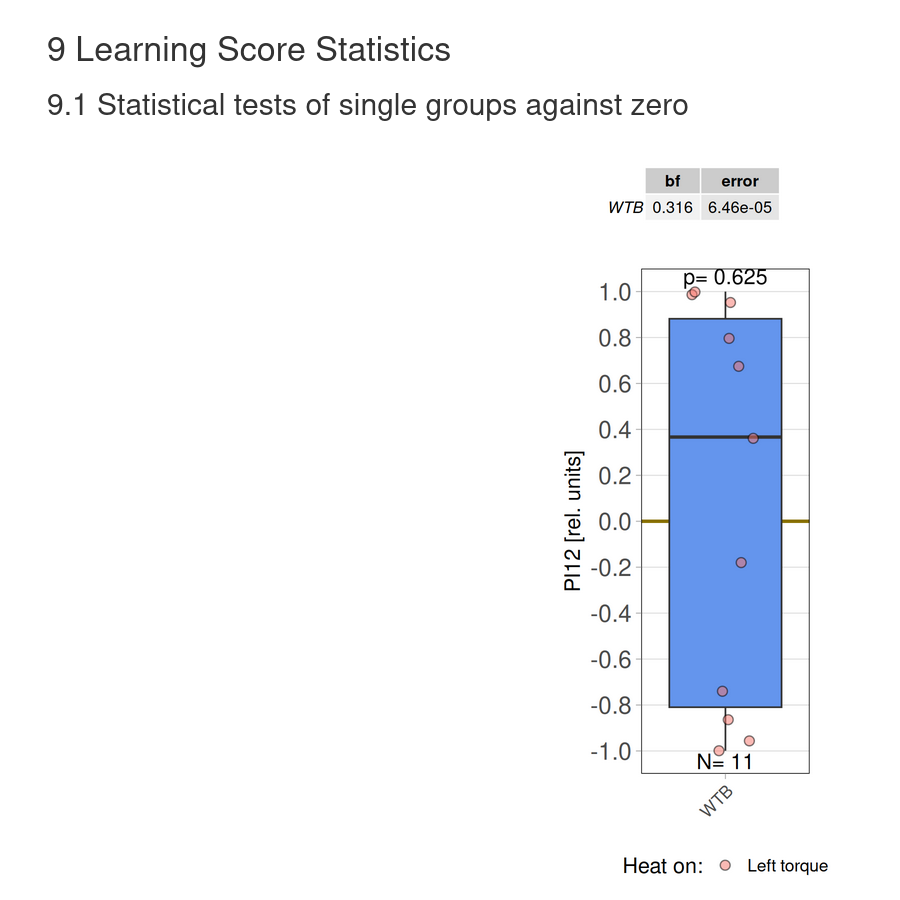
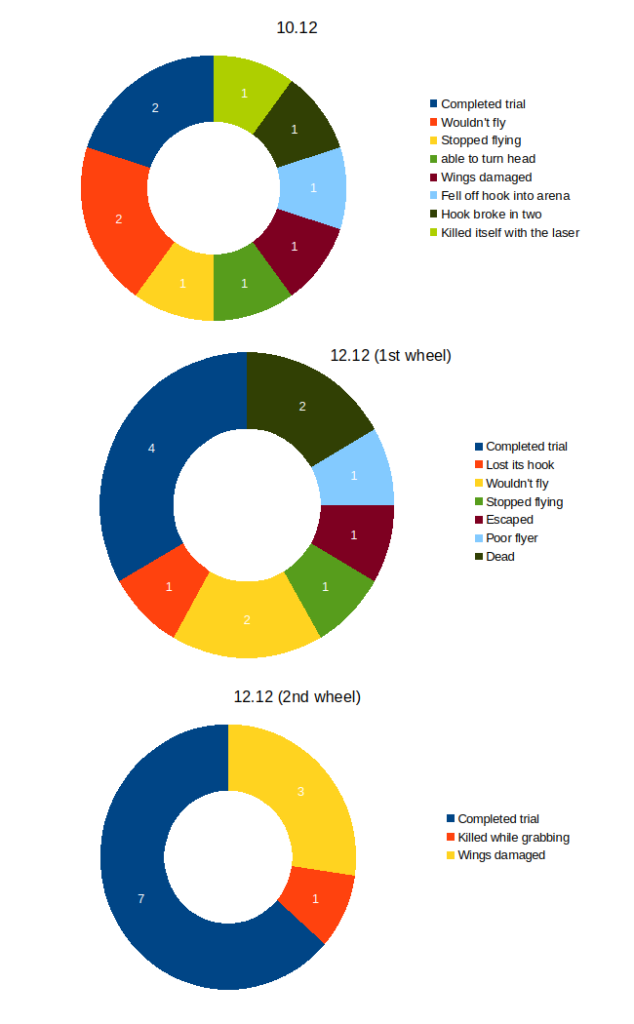
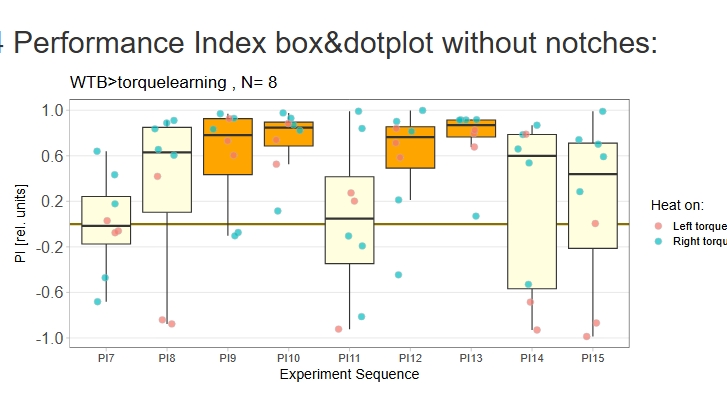
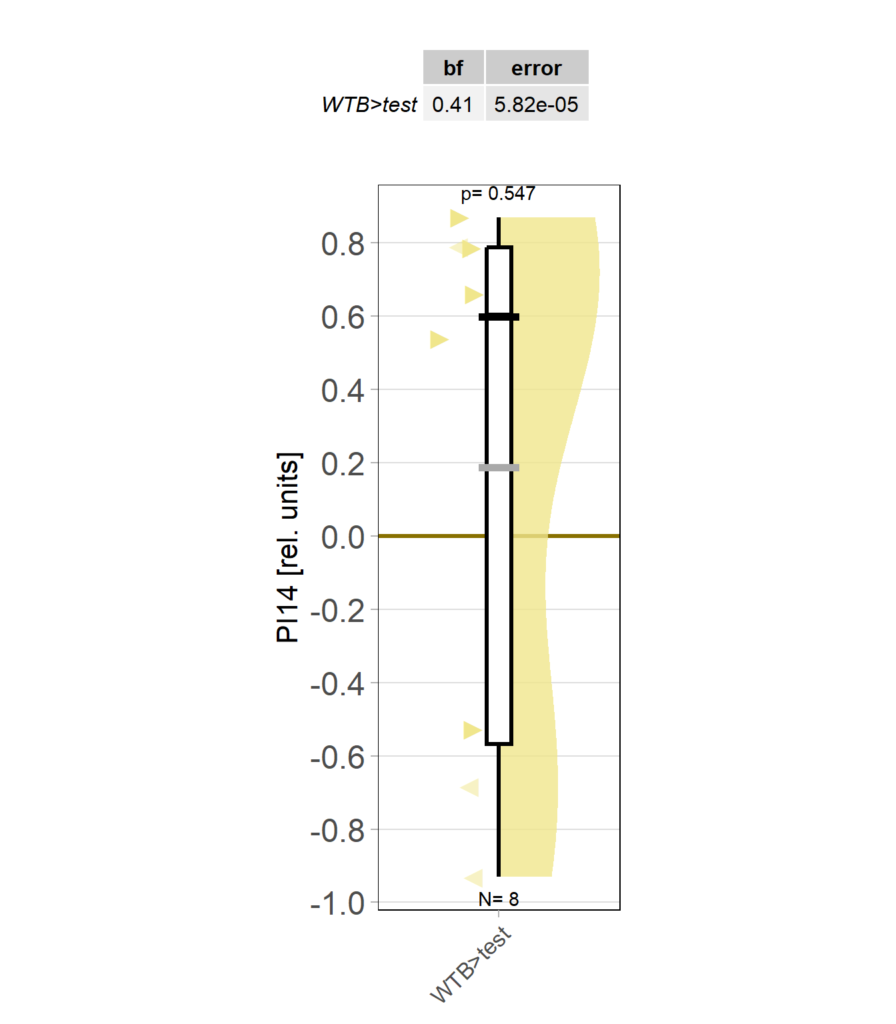
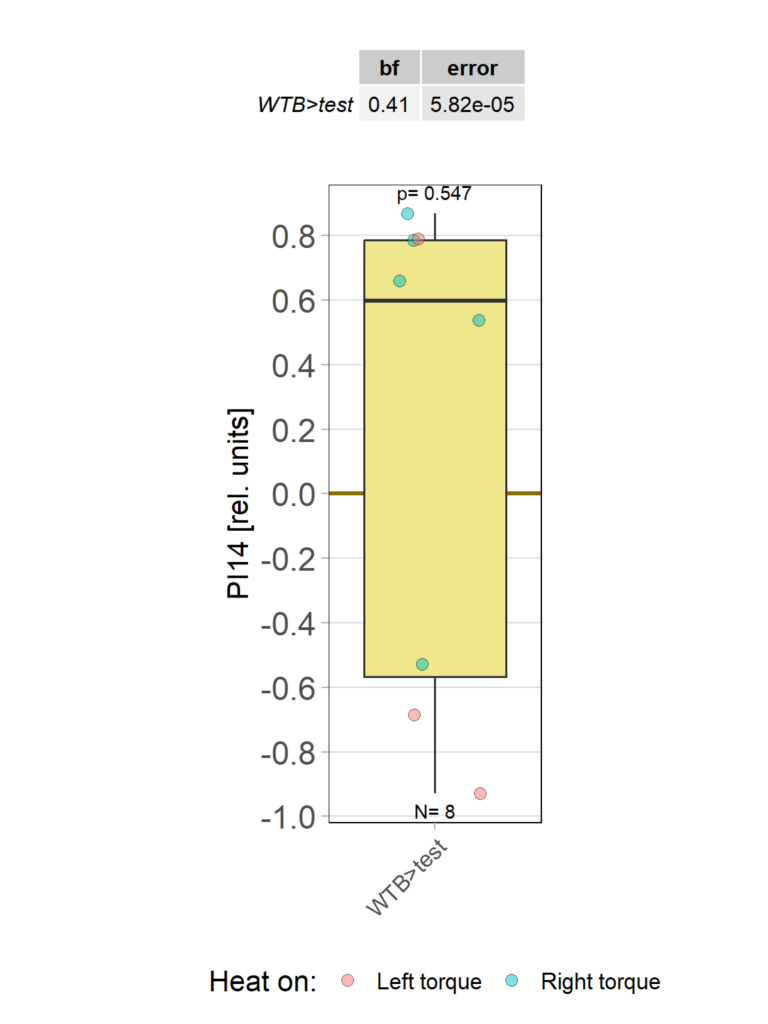
Torque learning results 12.12.2025 FBK
on Monday, December 15th, 2025 10:52 | by Fridrik Kjartansson
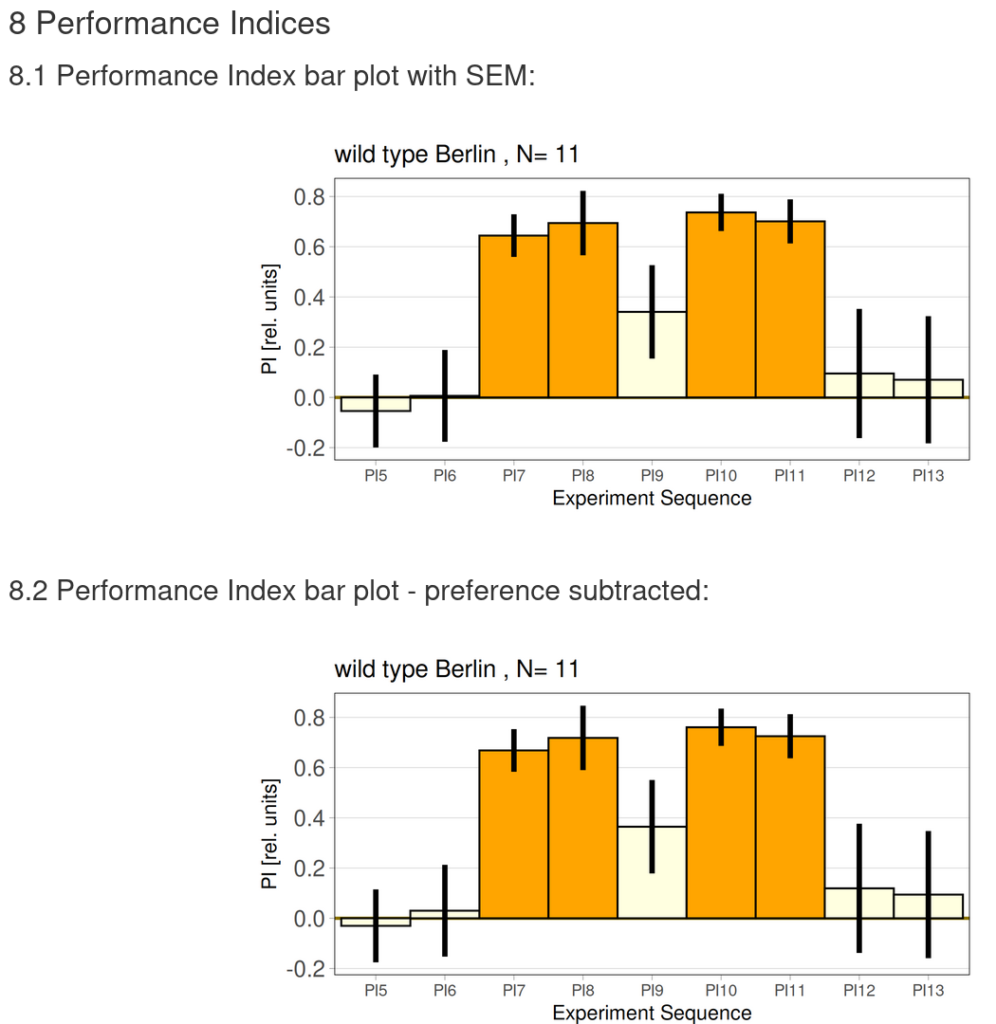
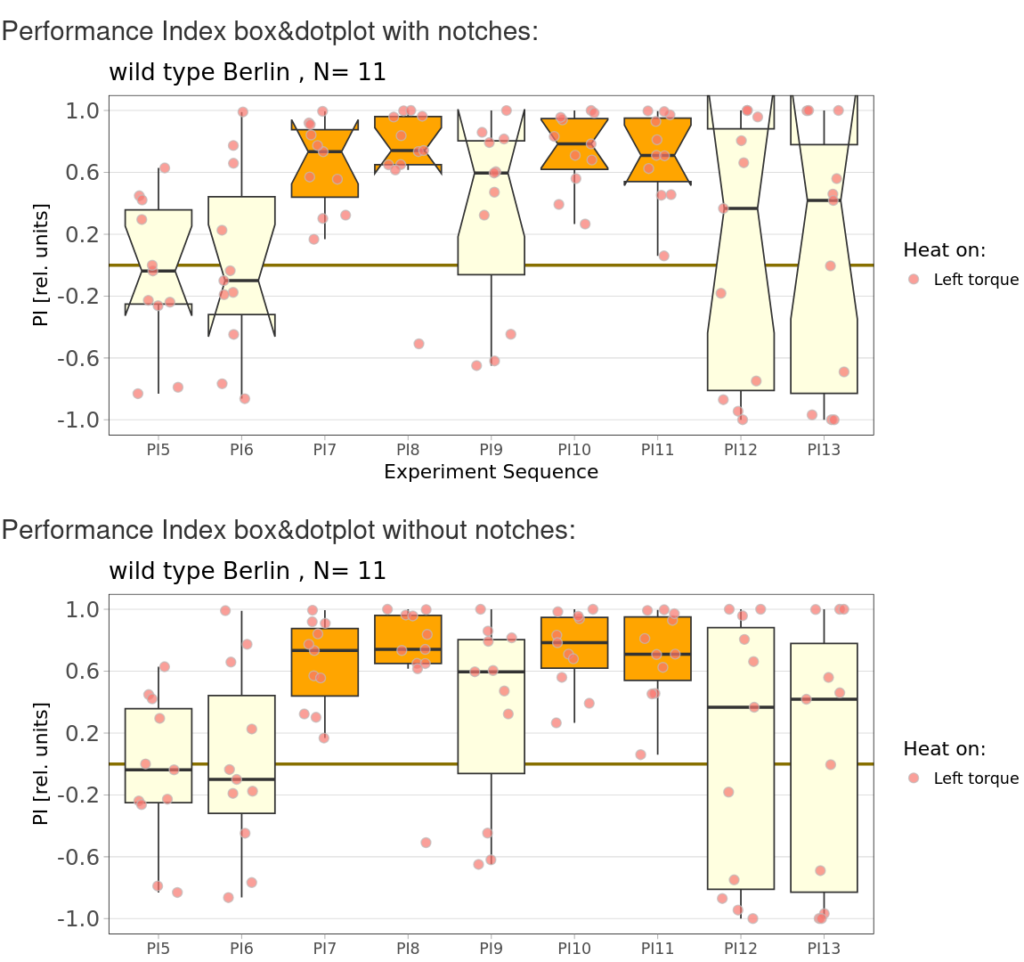
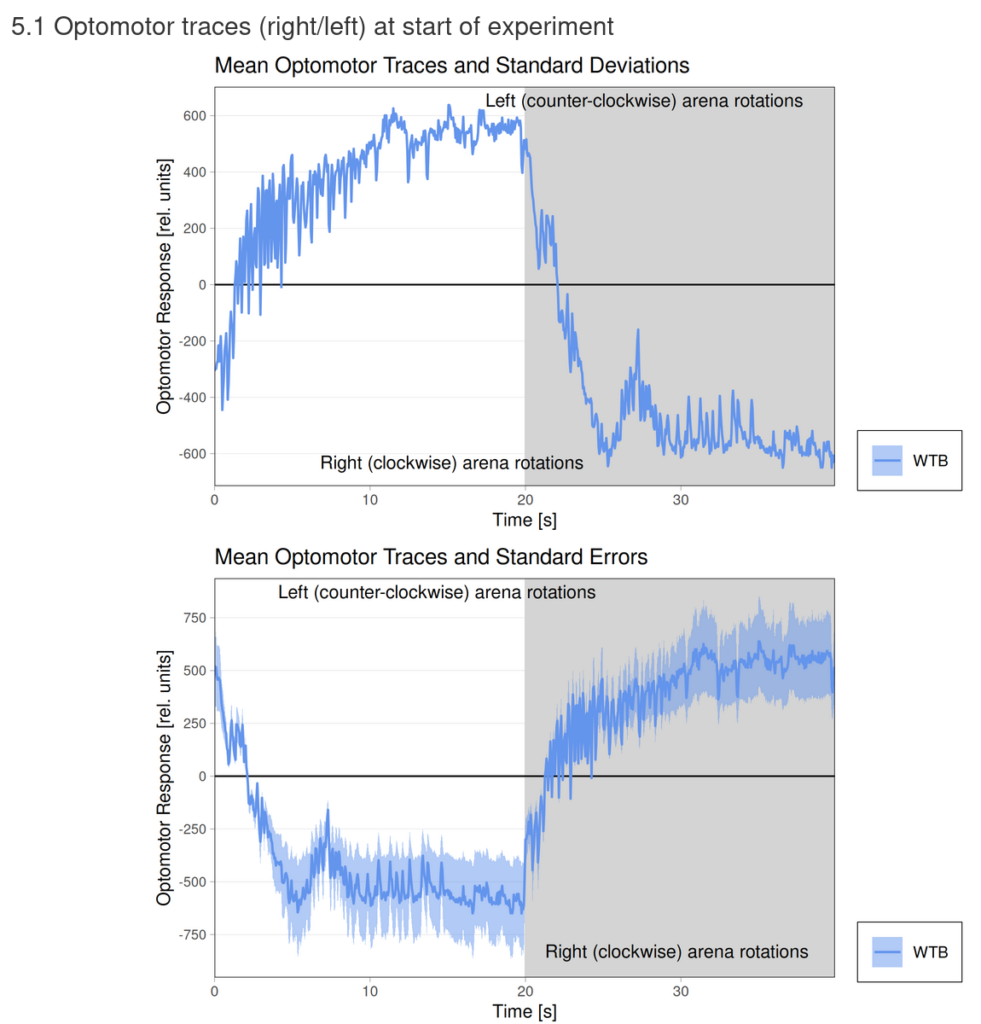
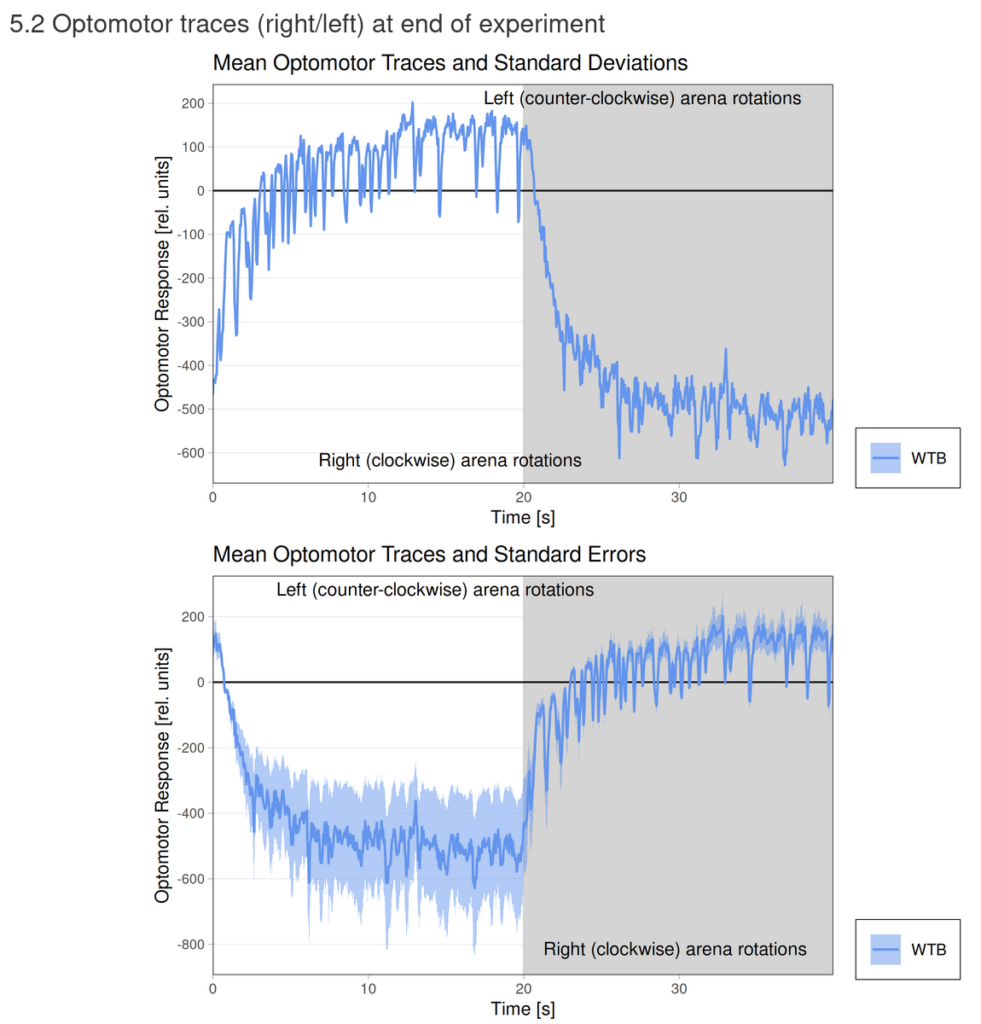
Category: flight, Operant learning, operant self-learning, Optomotor response | No Comments
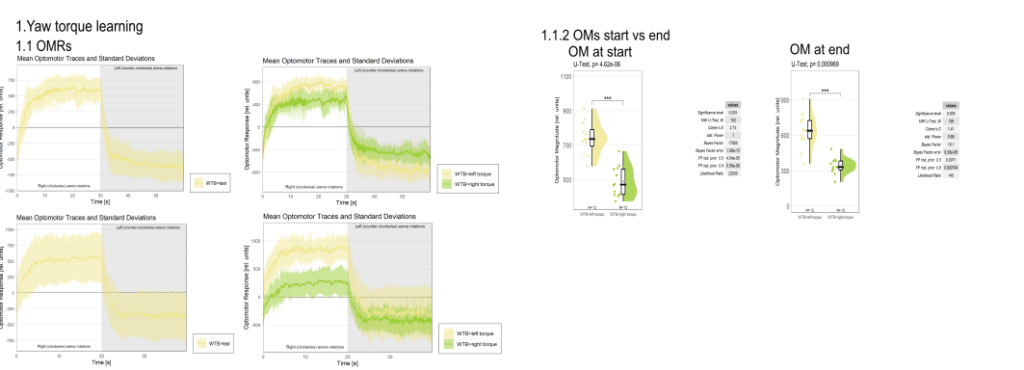
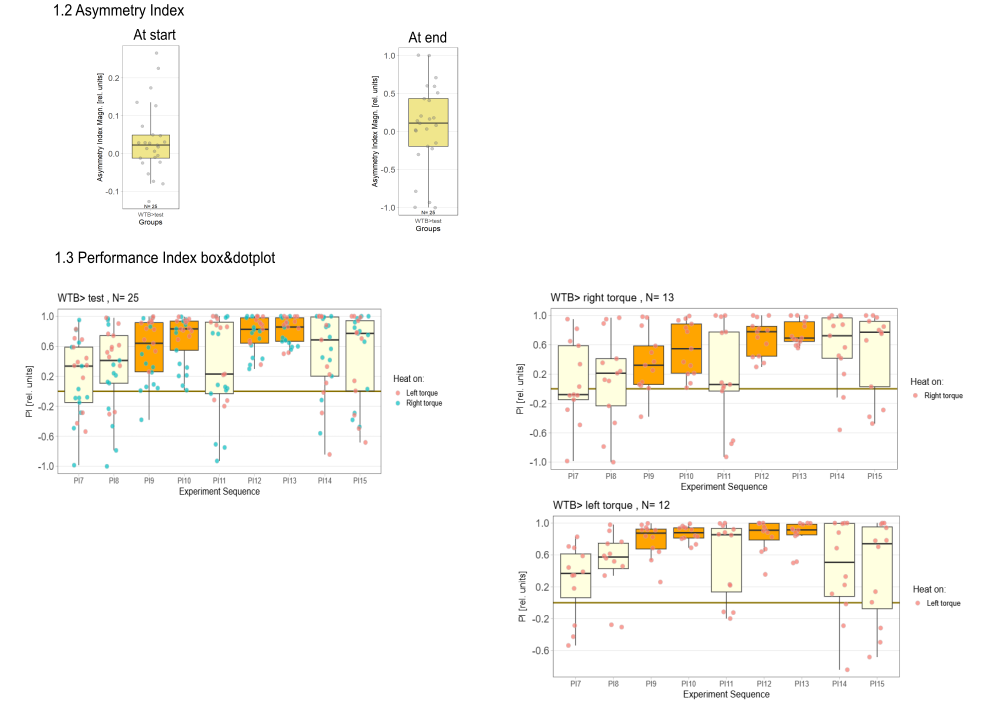
Yaw torque learning
on Monday, August 4th, 2025 1:57 | by Julia Schulz
Category: Operant learning, operant self-learning, Optomotor response | No Comments
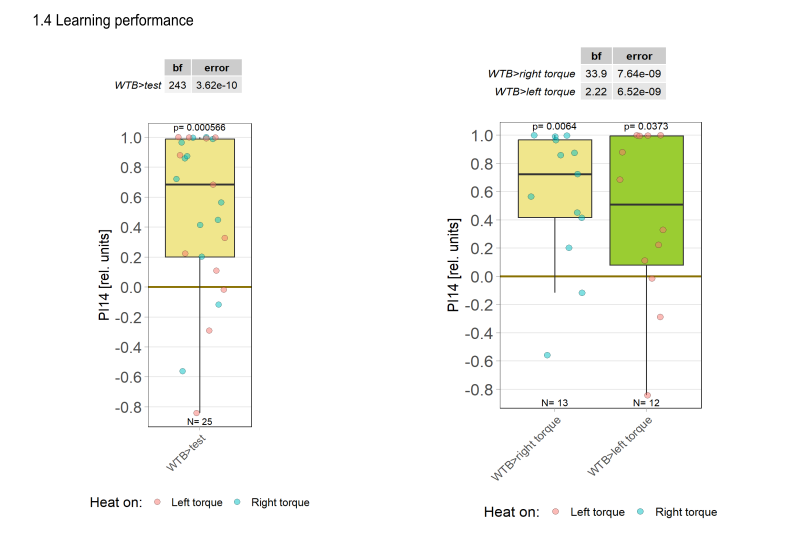
Optomotor test results for the first 3 crosses
on Monday, August 4th, 2025 1:54 | by Ece Kazgan
Category: Optomotor response | No Comments
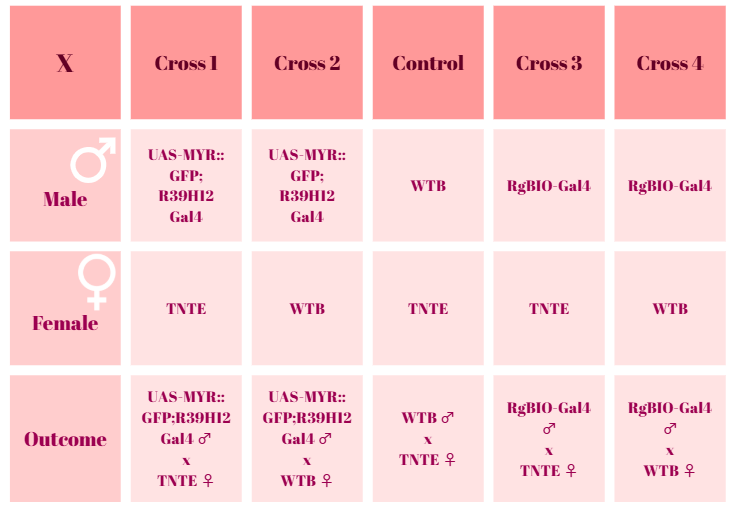
Crossing scheme for optomotor experiments
on Monday, July 28th, 2025 1:32 | by Ece Kazgan
After the 10th day, cross 2 and the control group hatched new flies. On the 13th day, all of the groups have hatched flies. None of the crosses seems to be lethal.
Category: crosses, Optomotor response | No Comments
yaw torque measurement_Dtc 20%
on Monday, April 14th, 2025 1:20 | by Julia Schulz
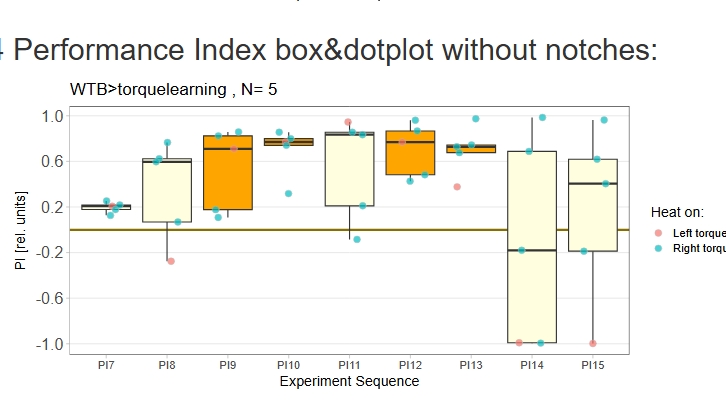
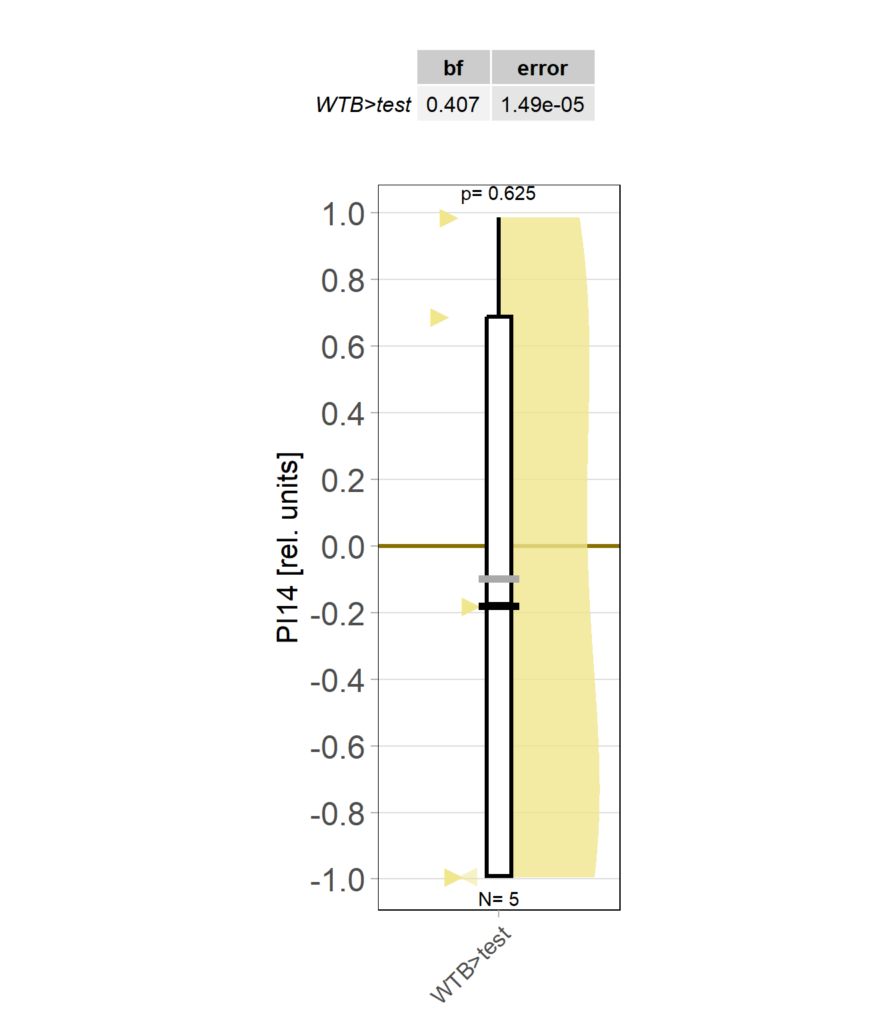
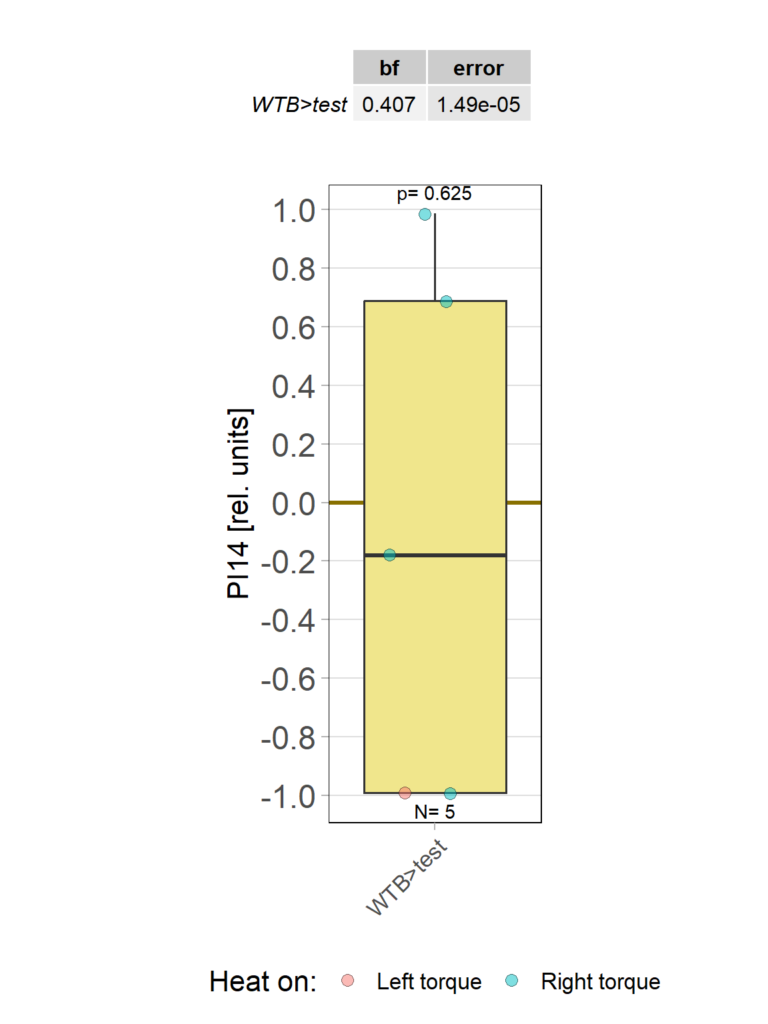
Category: operant self-learning, Optomotor response | No Comments
operant self learning_Dtc 40
on Monday, April 14th, 2025 1:13 | by Julia Schulz
Category: operant self-learning, Optomotor response | No Comments
It’s alive!!
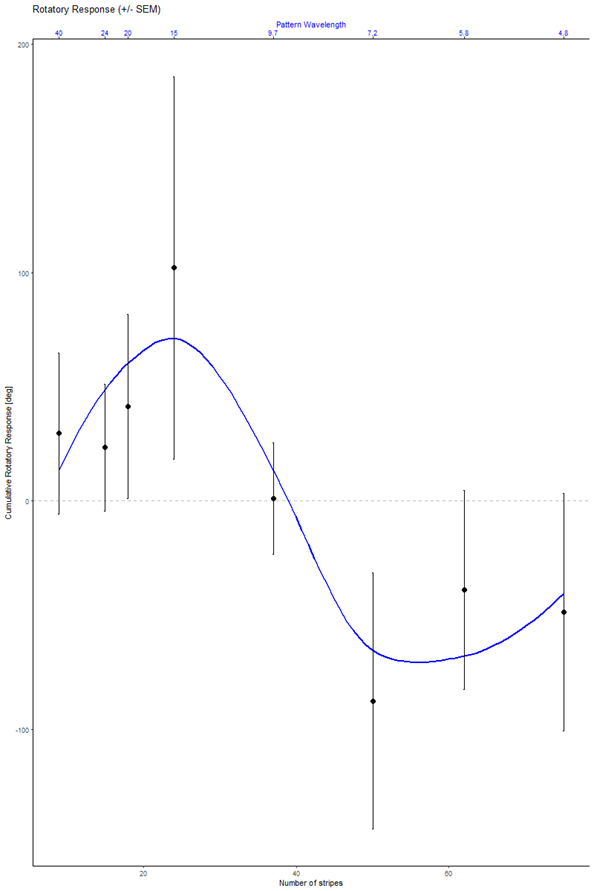
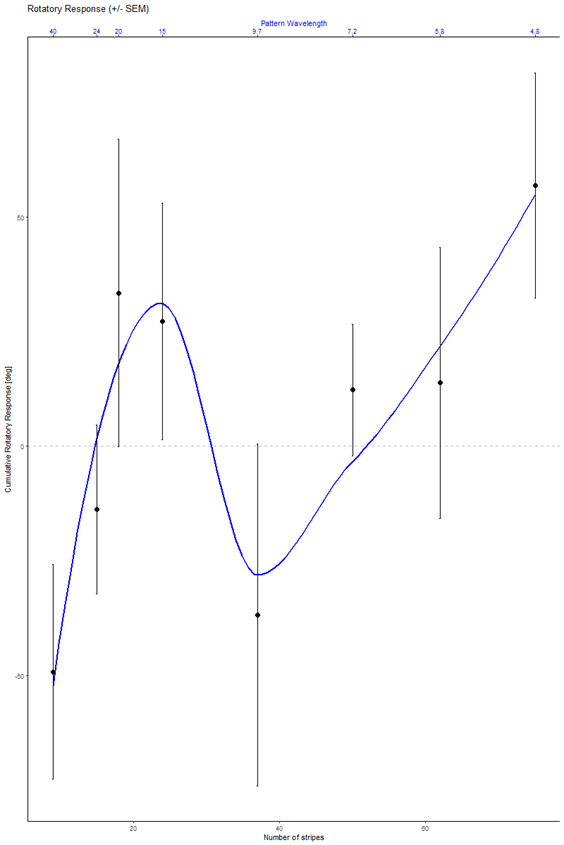
on Thursday, April 10th, 2025 1:31 | by Björn Brembs
Last week, Pavan Kaushik and I finally got the VR-panel setup working. I placed four flies in the machine, one in each VR cube, and then ran each fly three times with a sequence of 8 different pattern wavelengths, also repeated three times, each one rotated both counter-clockwise and clockwise. This is also the sequence which we will use in the course. This is what the data should look like:
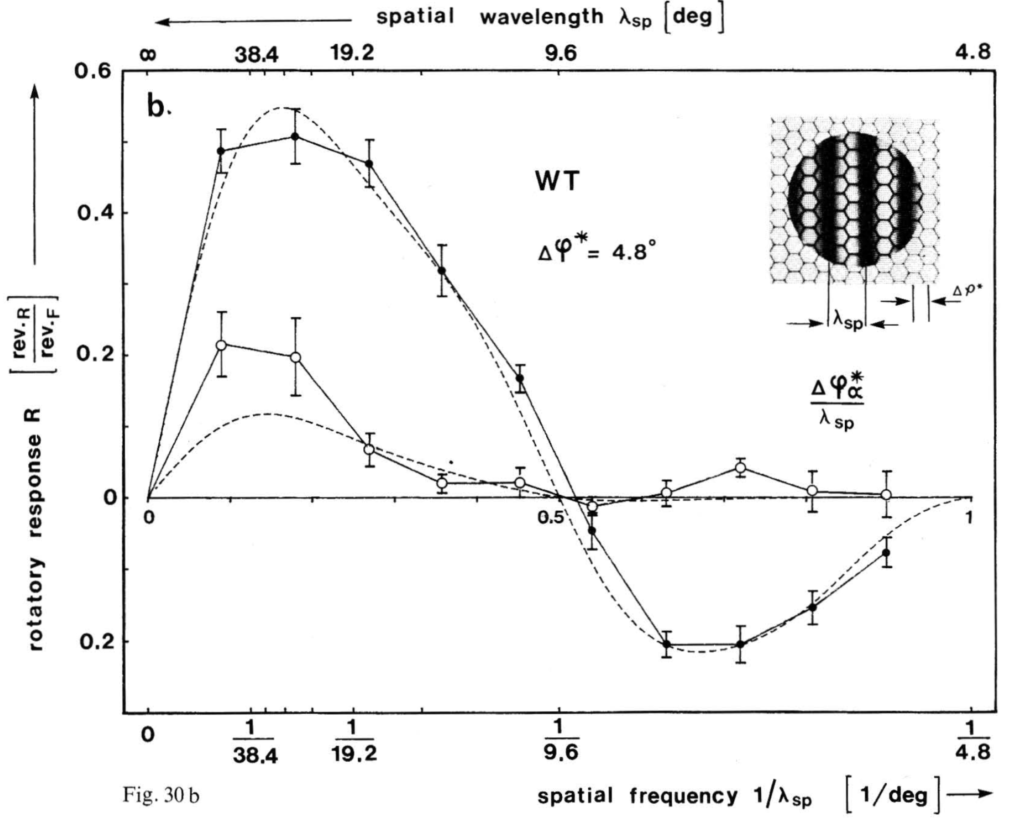
and this is what our data looked like:
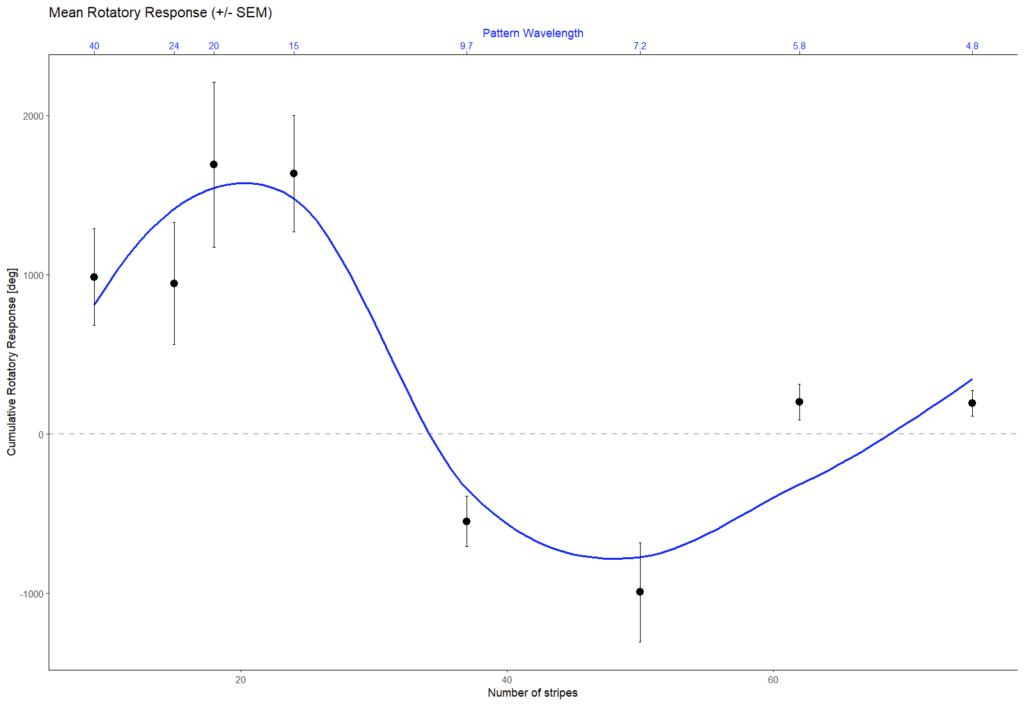
Looks to me like we got it working on the first try. Love that data. Great example to compare the course data against.
The code for evaluating the VR-Panel data is on GitHub.
Category: Optomotor response | No Comments
Strange results after pooling data
on Thursday, December 19th, 2024 3:55 | by Björn Brembs
Because the effect of yaw torque training on optomotor responses (OMRs) is still very small for now (we work on improving that), I pooled the two groups in which aPKC was knocked out in either motor neuron (MN) b1 or MN b3, as both these two groups and their WTB x aPKC/Cas9 controls seem to learn just fine (torque preference text after 8 minutes of training):
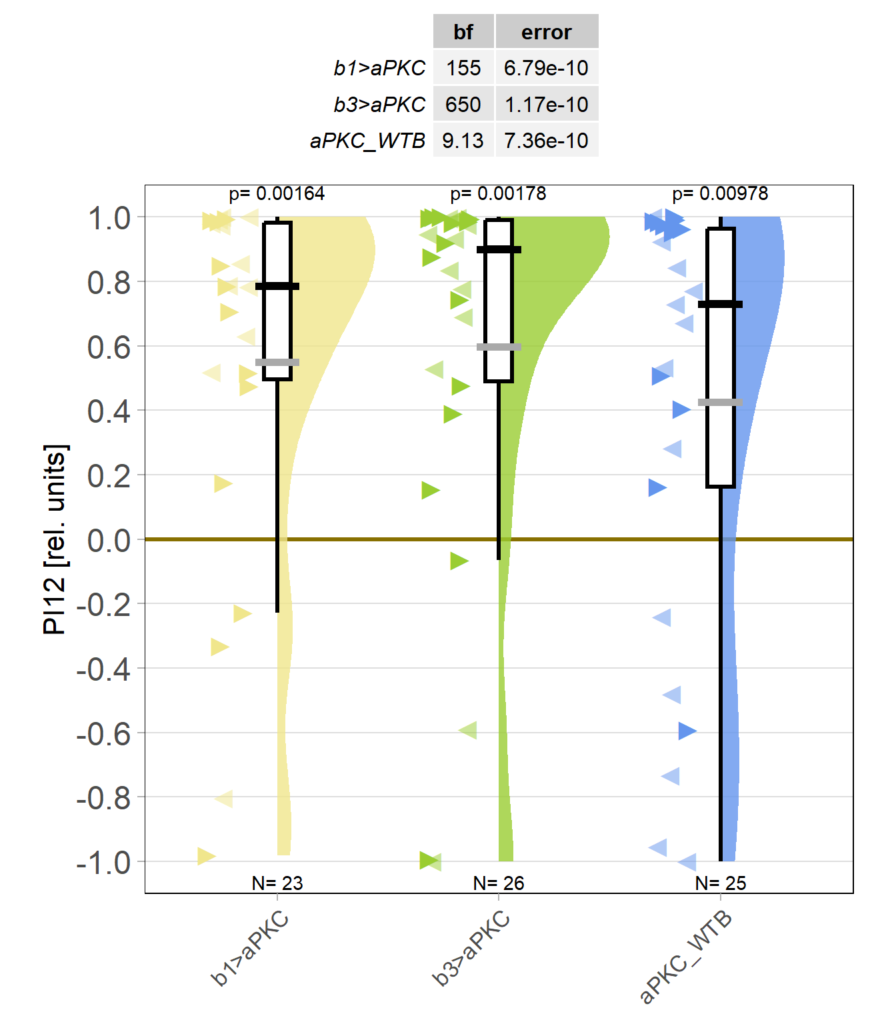
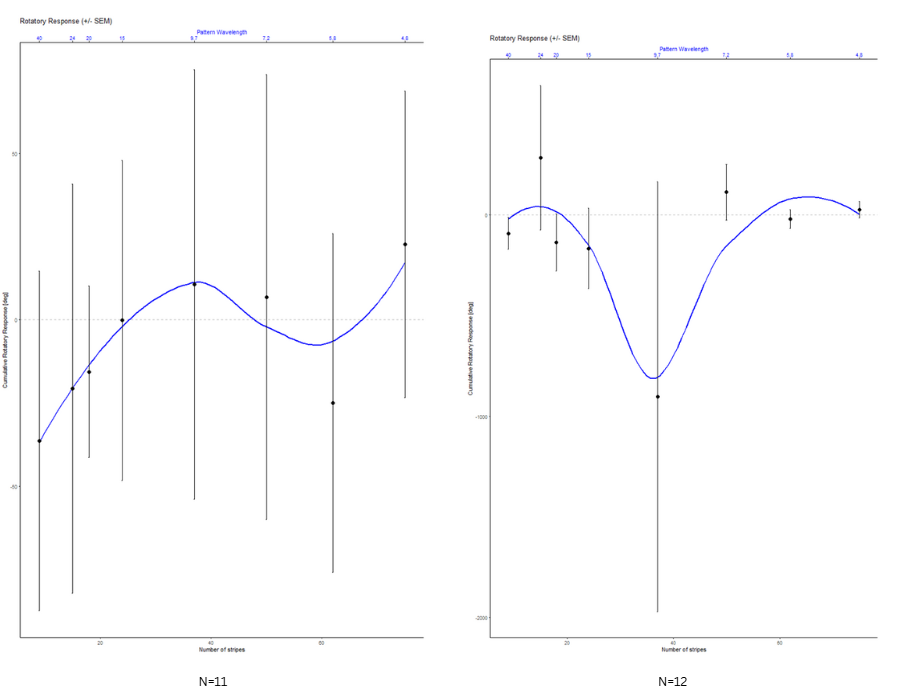
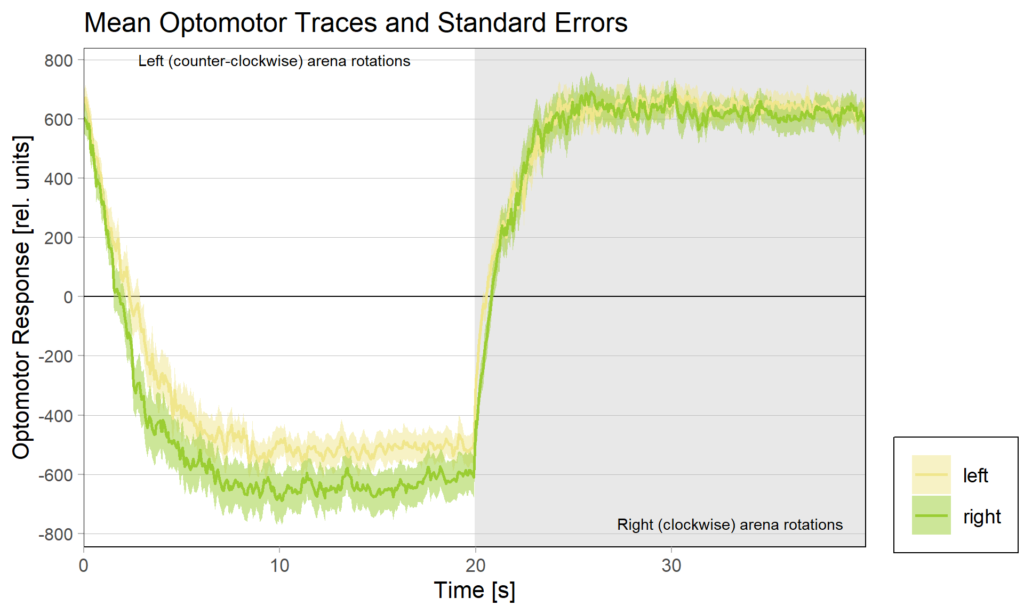
Obviously, we still need to check the Gal4 driver lines are really targeting the right neurons, but assuming they are ok, it seems like neither an aPKC knock-out in b1 alone nor in b3 alone is sufficient to affect operant self-learning. Maybe this is due to b1 and b3 acting as an agonist/antagonist pair and if one of them fails to show plasticity, the other is sufficient on its own? Another explanation could be that the torque preference depicted above is mediated by other neurons than b1 or b3 and that the OMR modulation is gone in these flies. Because the OMR effect is so small, I pooled the two groups, threw out all flies that didn’t have at least an acceptable OMR and halfway accurate OMR parameter estimation and plotted the OMR traces of the remaining 35 flies after training:
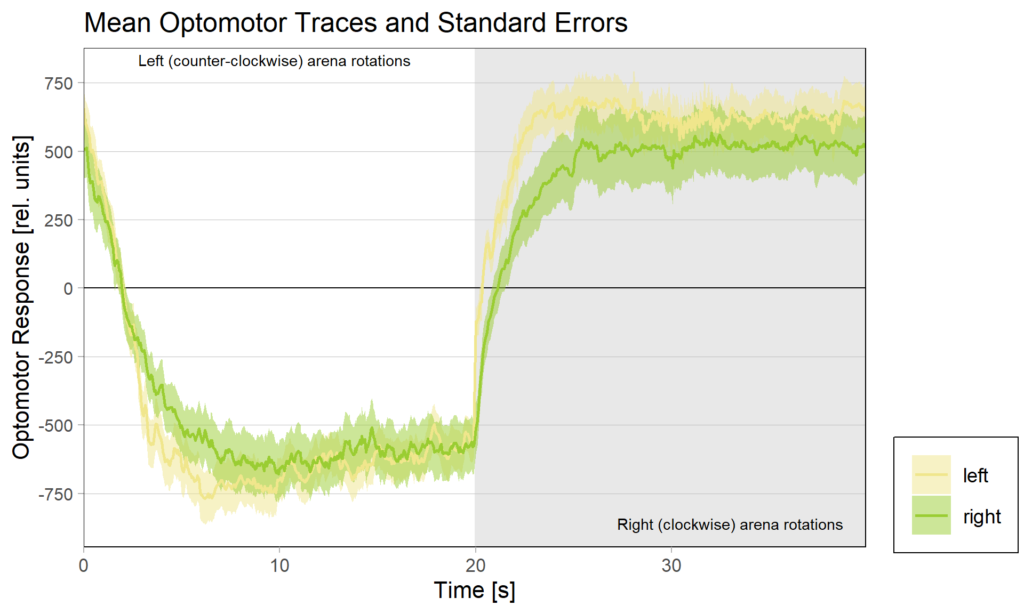
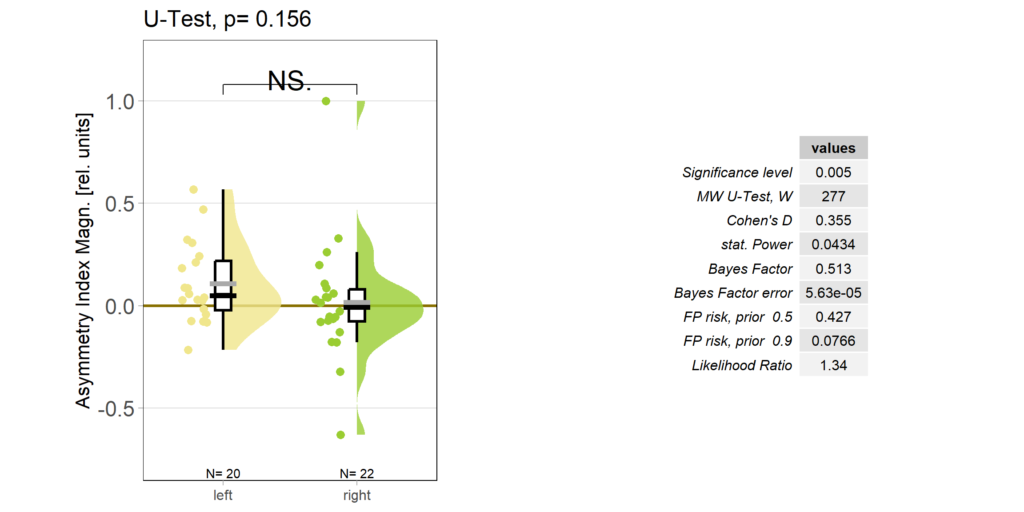
So despite these flies learning well, the OMR does not seem modulated as one can see in WT flies. However, there my be a slight effect for the fly punished on right turning torque, perhaps? However, this group also has much larger errors, which I would need to check the reason for. The quantification of the OM symmetry does not show any hint of an effect, though:
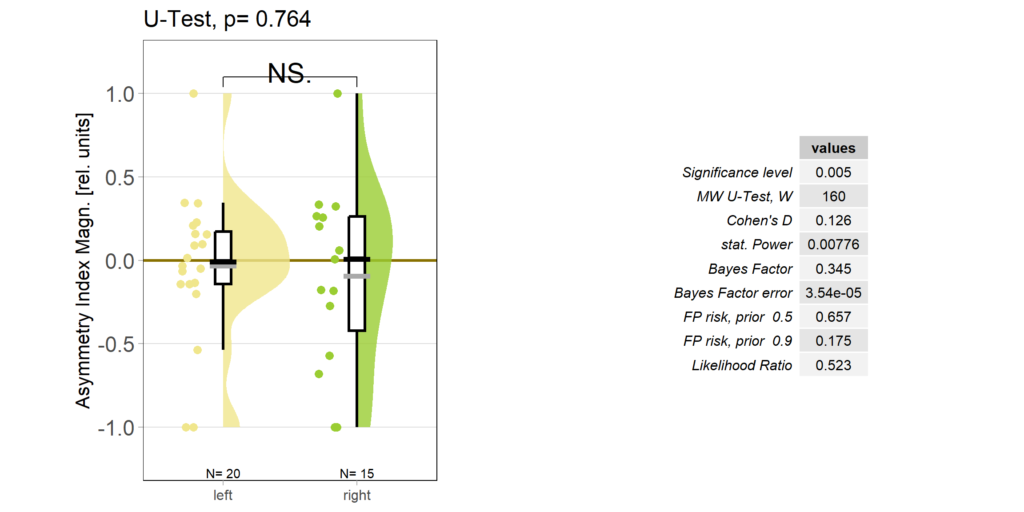
Below the total evaluation before and after training. What is weird is that despite there being no effect after training, the correlation between torque preference and OMR asymmetry seems to be there – or is it just the three outliers?
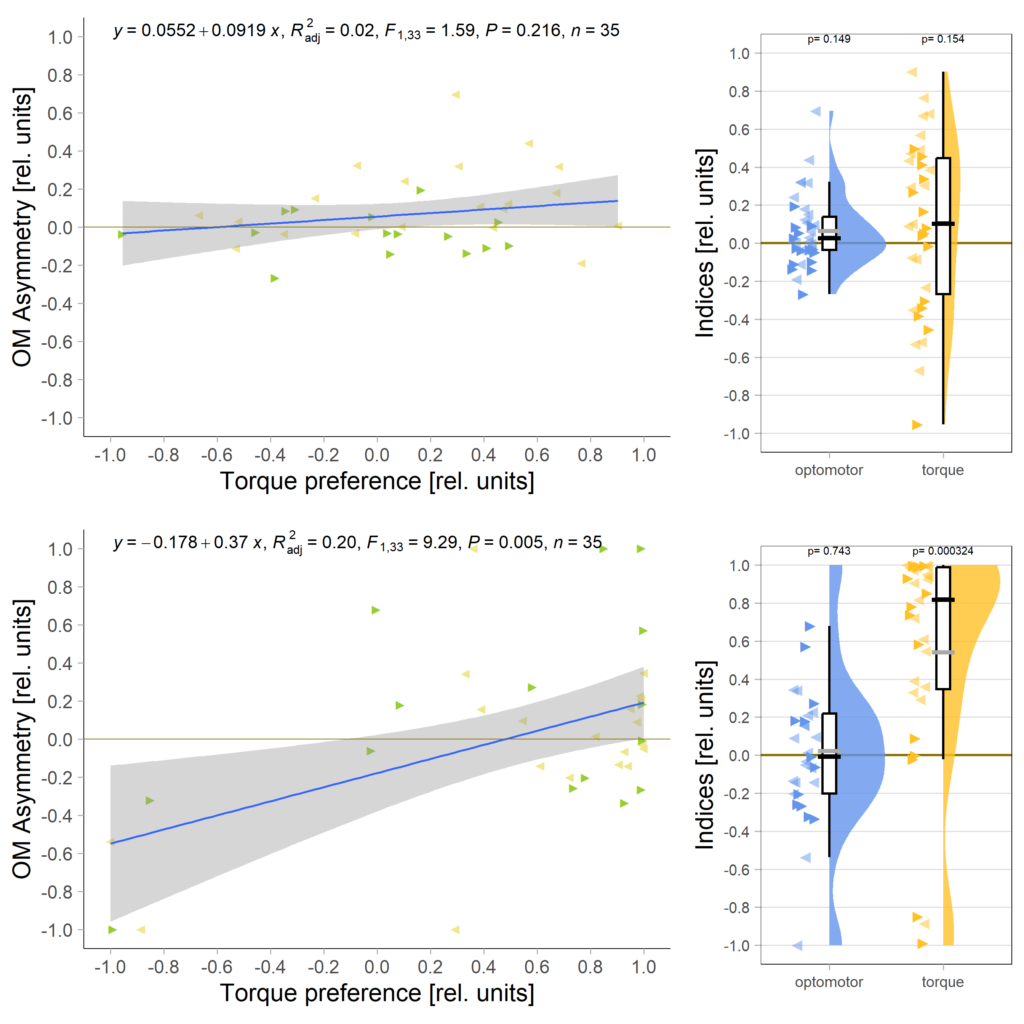
Either way, when I pooled the control flies from this experiment with the same genotype from the last experiment to get to 42 flies, only the group that was punished on left-turning torque showed the modulation:
Accordingly, the quantification shows no difference ion the control group either:
And no significant correlation between the indices either:
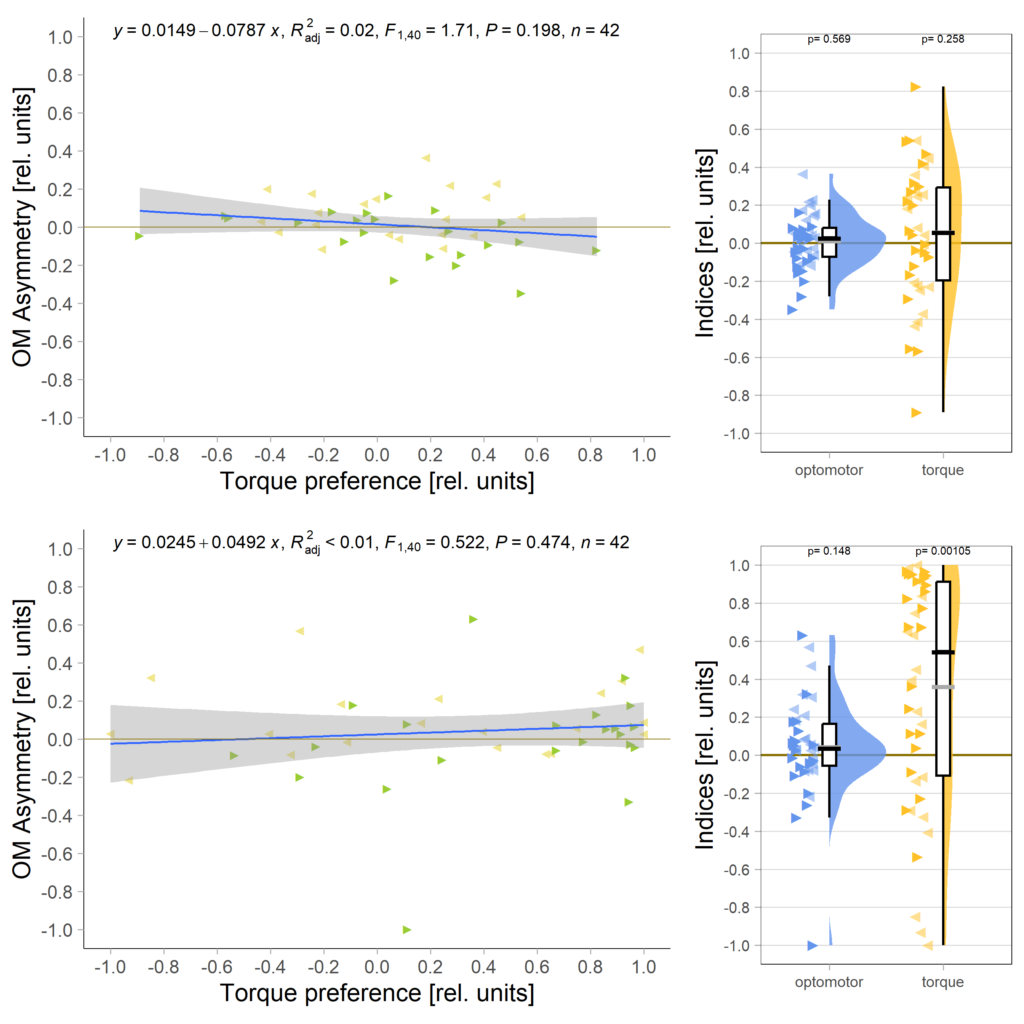
All in all rather puzzling results that reinforce my view that the OMR effect is much too small to practically work with. That means one of the next goals must be to get this effect size increased by, perhaps, decreasing the strength of the optomotor stimulus?
Category: operant self-learning, Optomotor response, PKC | No Comments
b1/b3 aPKC KO flies still learning, OMR unaffected
on Friday, November 22nd, 2024 3:47 | by Björn Brembs
Now with over 20 flies in each group, it becomes more and more apparent that both the flies without aPKC in either b1 or b3 steering motor neurons still learn just fine:

As with the aPKC knock-out in FoxP neurons, also here, the optomotor response seems normal as well:

Interesting is the scatter in the slope parameter for the control flies:

Category: operant self-learning, Optomotor response, PKC | No Comments
Almost there
on Monday, July 8th, 2024 8:33 | by Björn Brembs
Not many fliers left now. Will start evaluating optomotor asymmetry now.

Category: operant self-learning, Optomotor response, PKC | No Comments
