Breaking the ellipsoid body code
on Monday, October 20th, 2025 12:14 | by Björn Brembs
Now that I’ve reached a sample size of near 30 animals in each group, it was time to break the code to see which group was wich. To my surprise, the worst performing group in term,s of learning was the control group:
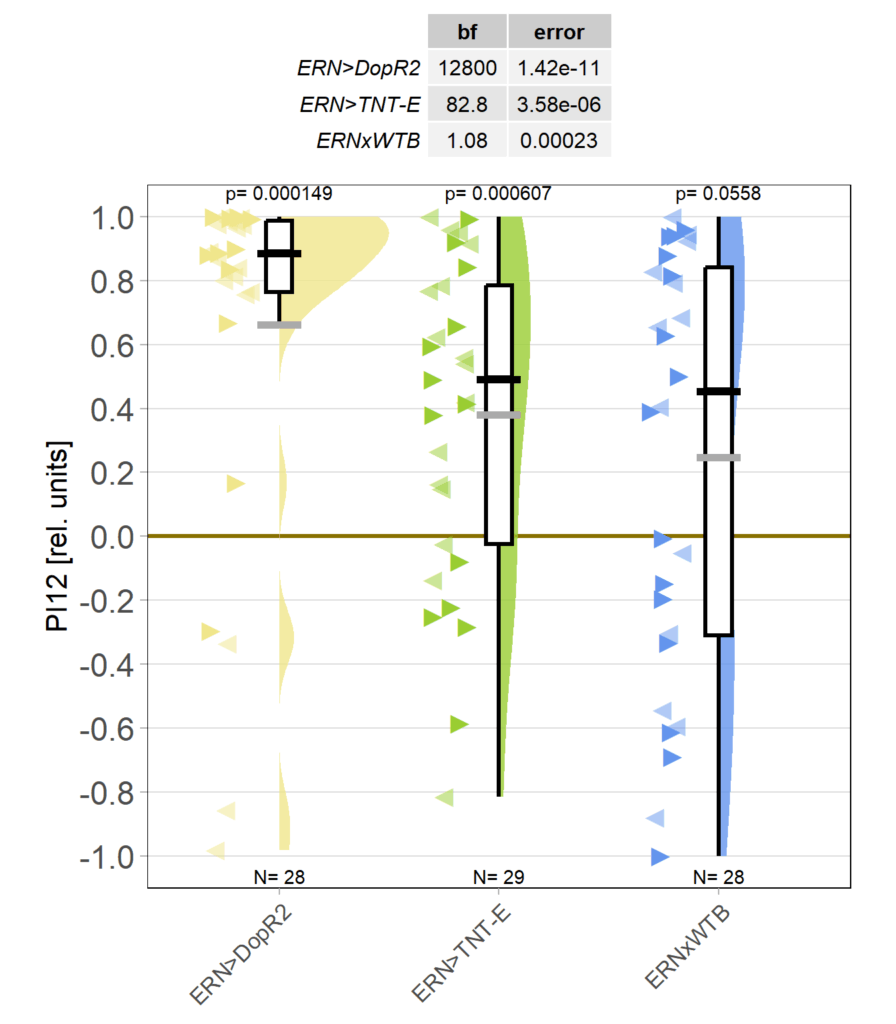
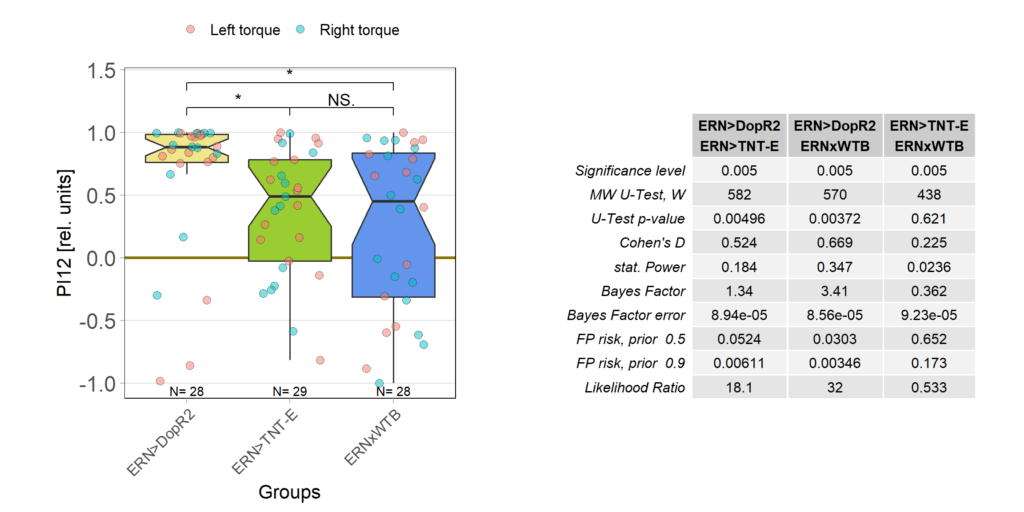
It is unusual for between-groups comparisons to become statistically significant at the 0.005 level with a sample size of less than 50, let alone 30, but these DopR2 knock-downs in ellipsoid body ring neurons are just a class of their own:
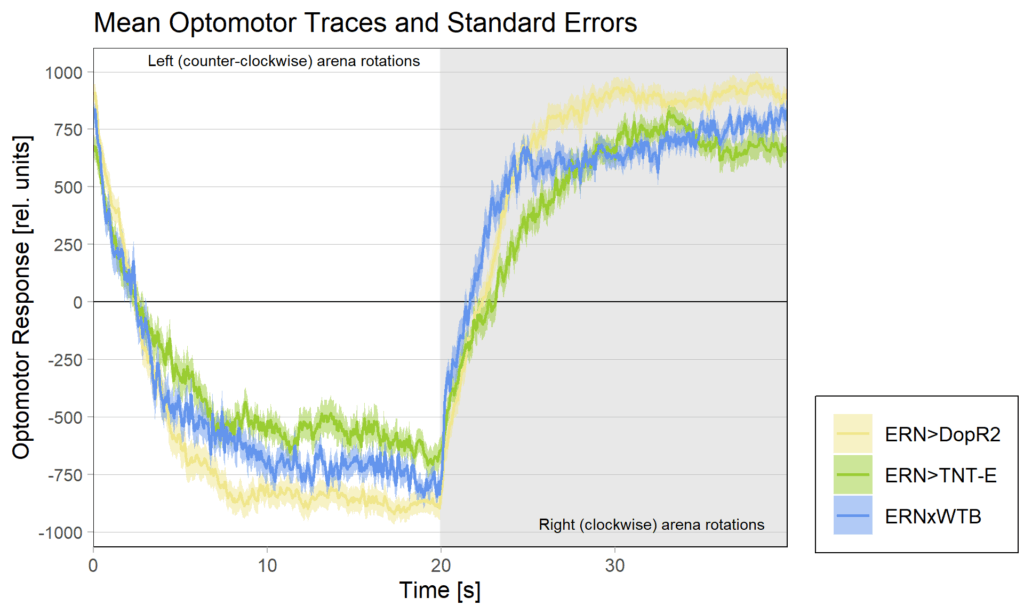
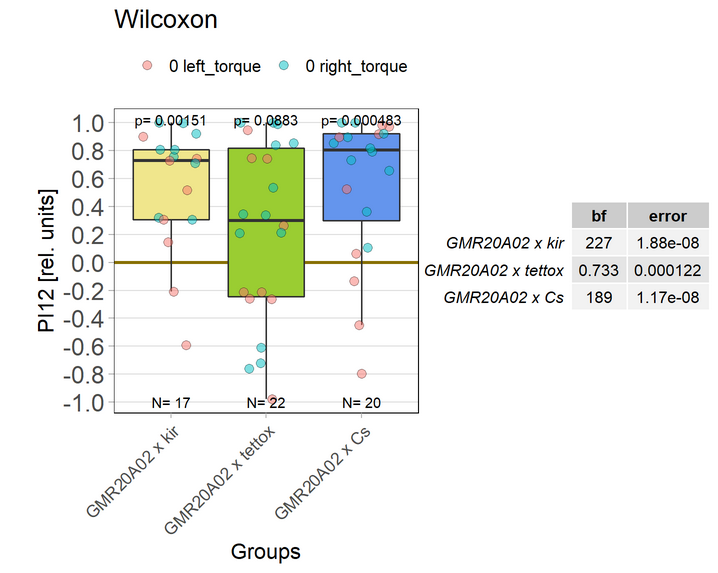
The DopR2 receptor knock-down not only performed exceedingly well in these leaning experiments, it was also the cross with the strongest optomotor response:
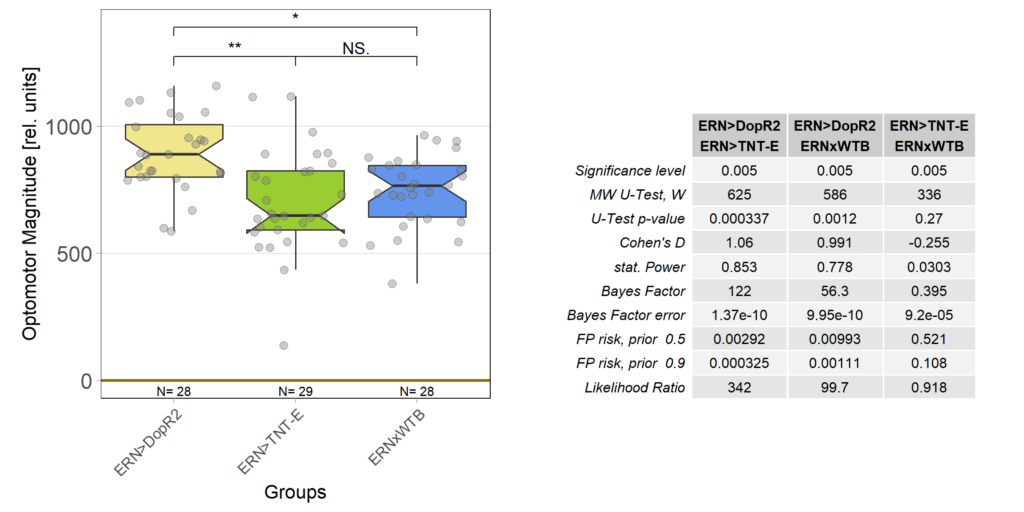
And also that difference is statistically significant:
The TNT-E flies had a slight positive preference, worse avoidance (esp. in the first training period) and flew really bad. One could say they did have some learning deficits, but it doesn’t look as if learning was completely abolished (which is roughly what Andreas had found before):
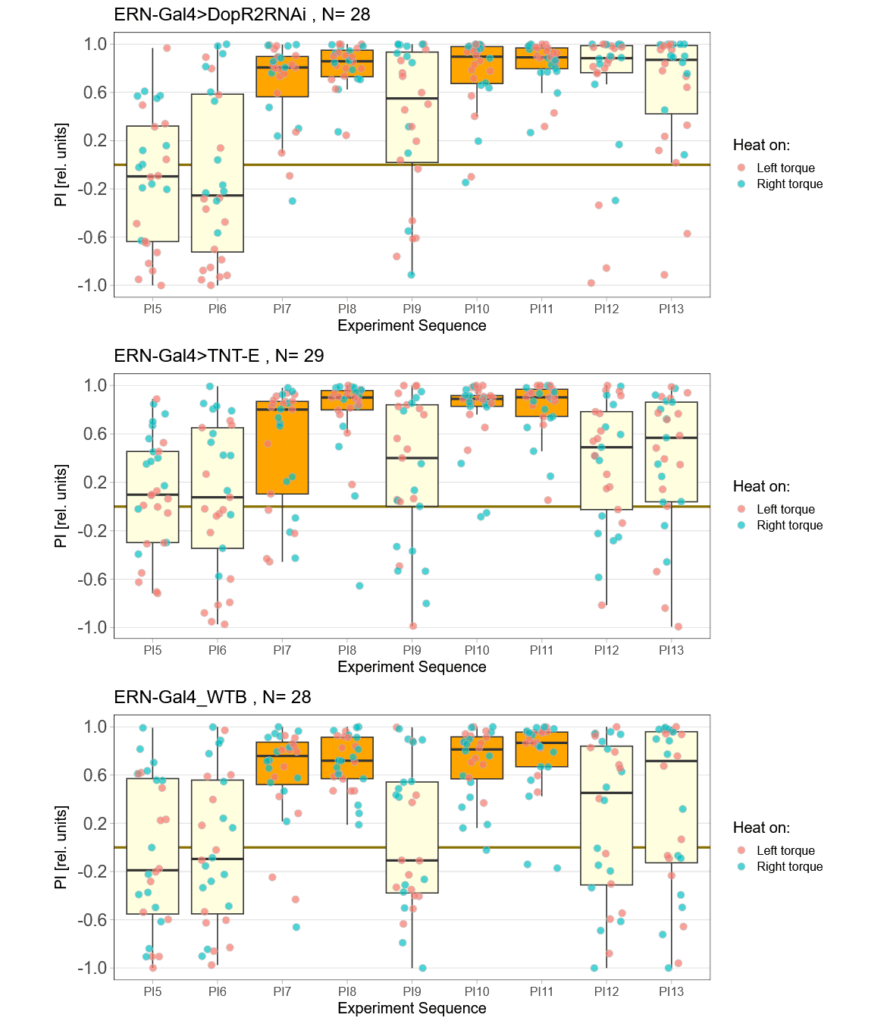
Andi’s results:
Category: operant self-learning | No Comments
Almost there now…
on Tuesday, October 7th, 2025 9:57 | by Björn Brembs
Getting close to a sample size of 30! One, maybe two weeks left, depending on how much time I find to measure. Time to break the code is drawing nearer. Here the current results:
Some interesting statistics:
Category: operant self-learning | No Comments
2.Yt learning red line
on Monday, September 29th, 2025 1:01 | by Julia Schulz
2.1 OMRs at start
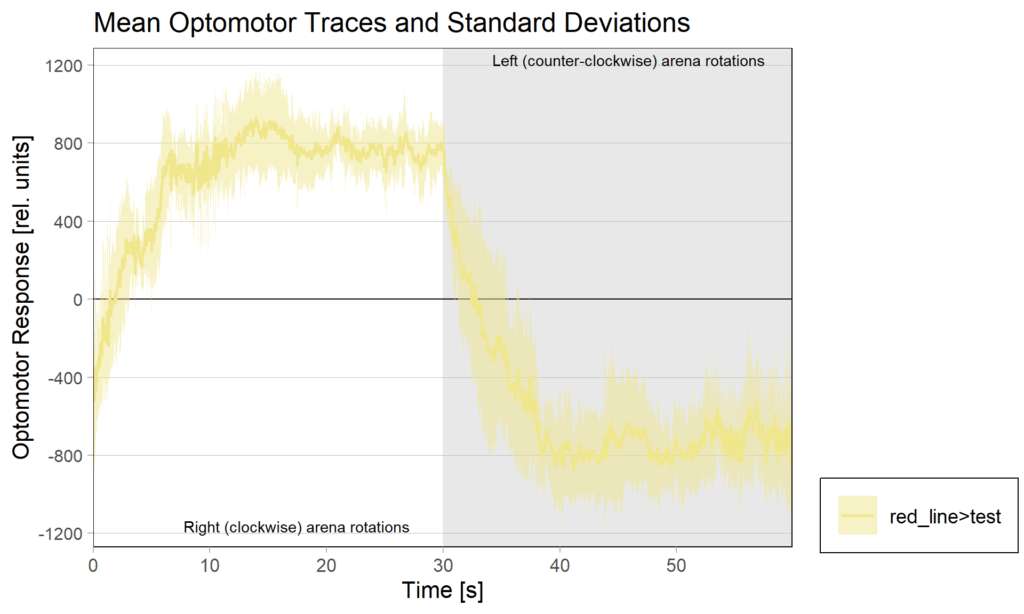
2.2 OMRs at end
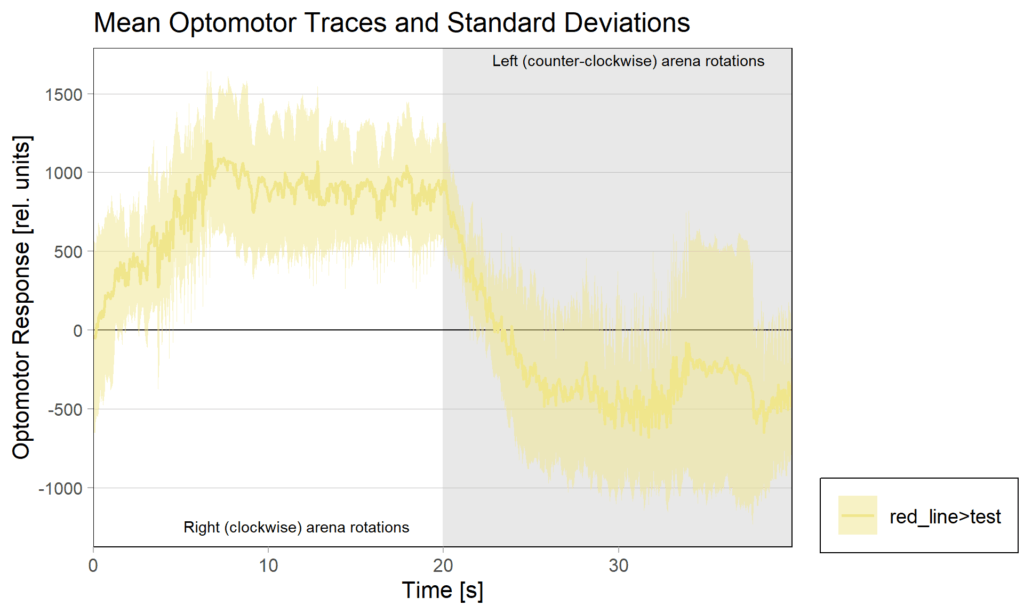
2.3 PI( preference subtractted)
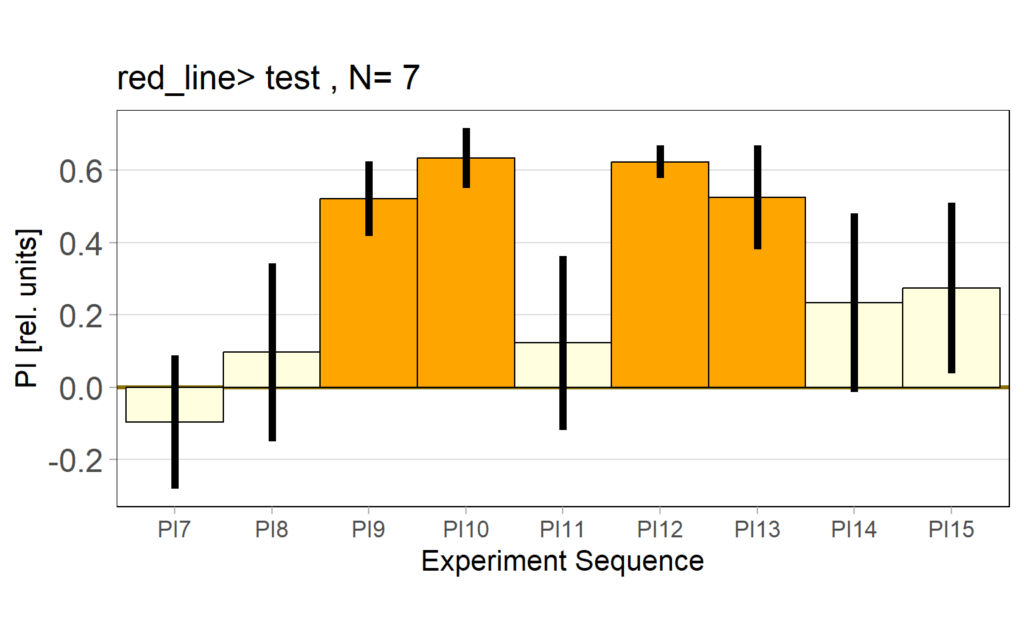
2.4 statistics
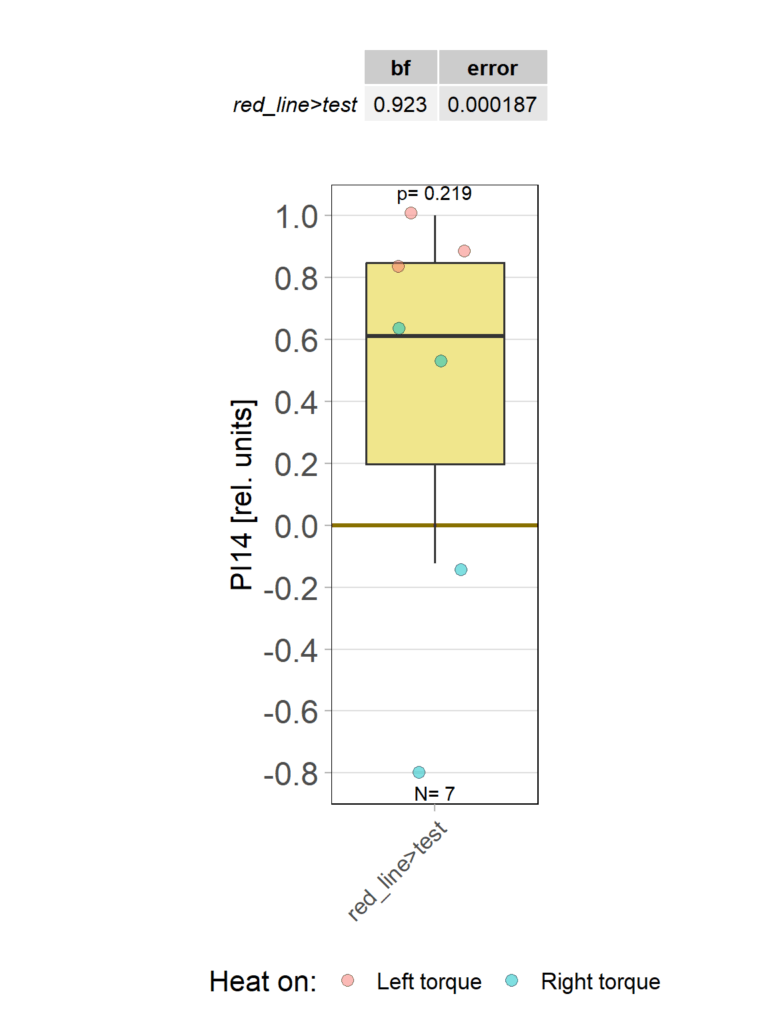
Category: Operant learning, operant self-learning | No Comments
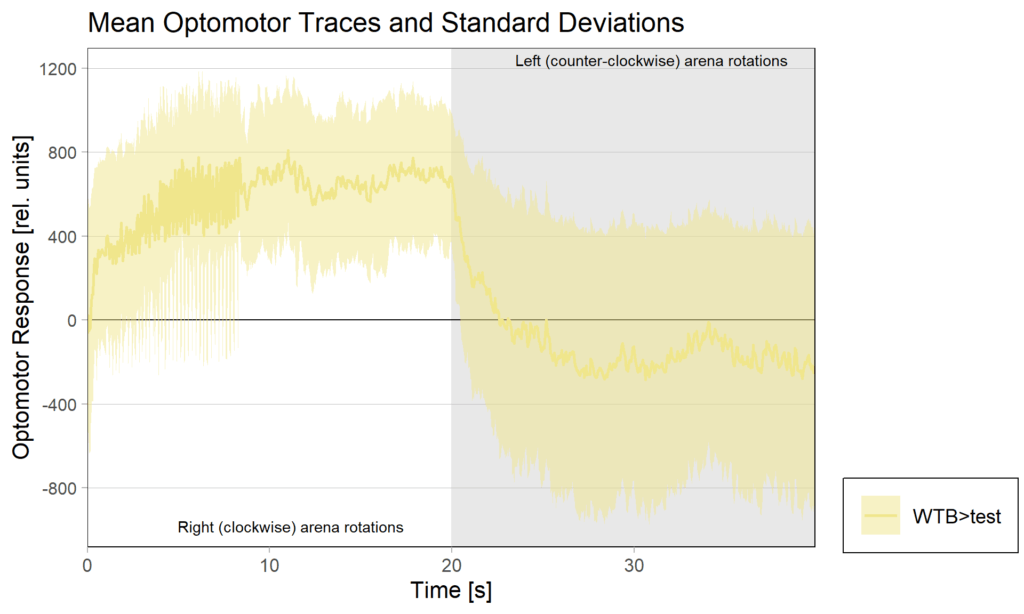
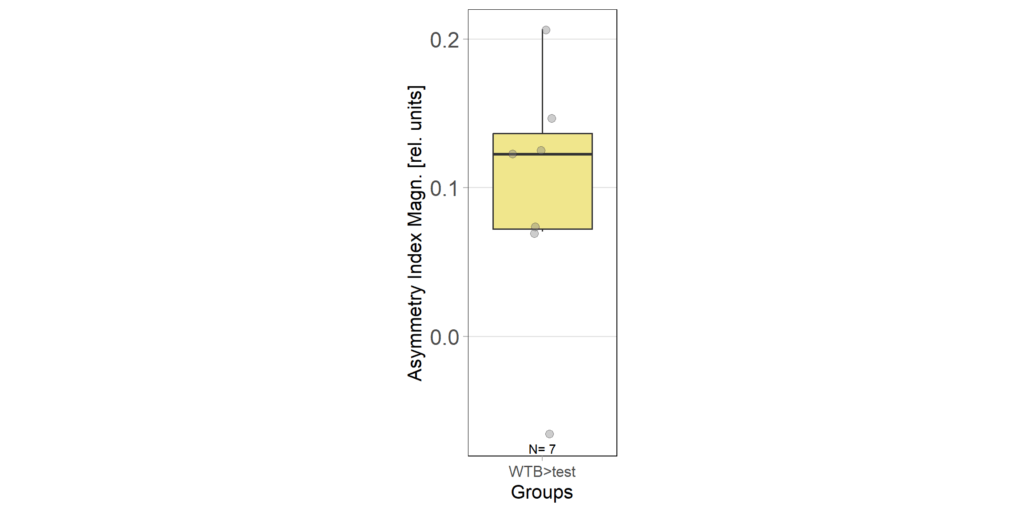
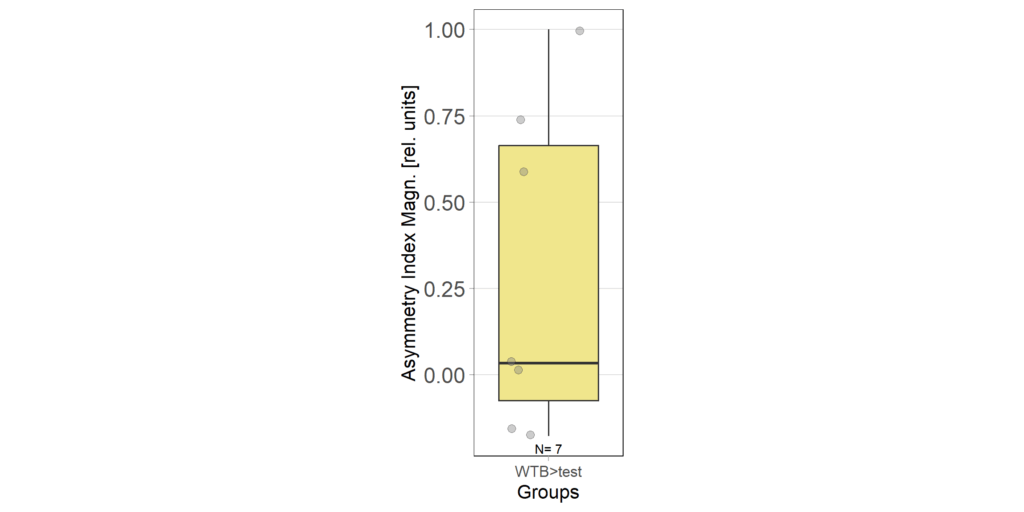
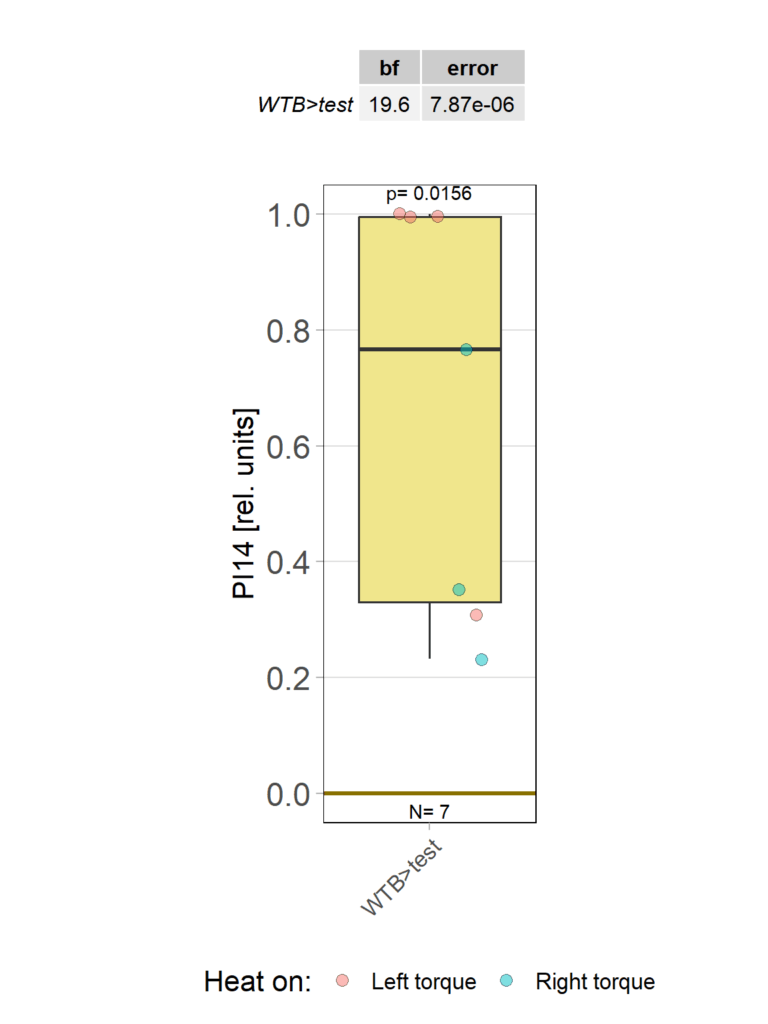
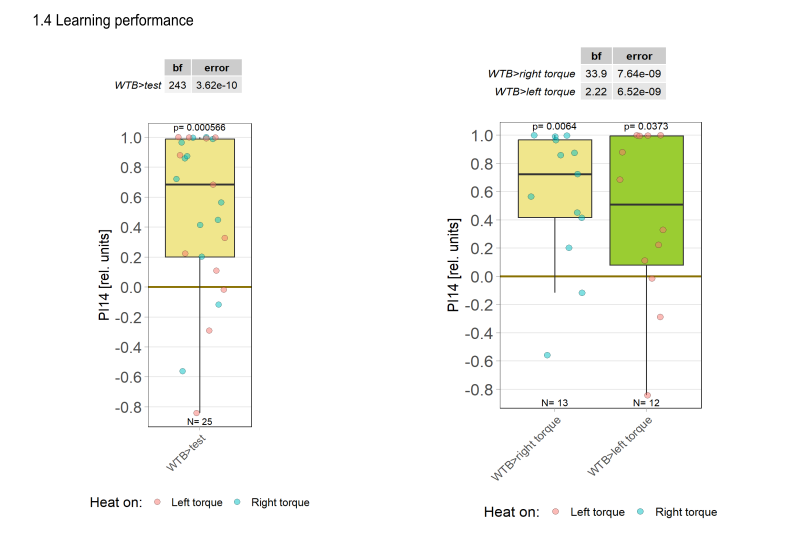
1.Yt learning_WTB flies
on Monday, September 29th, 2025 12:58 | by Julia Schulz
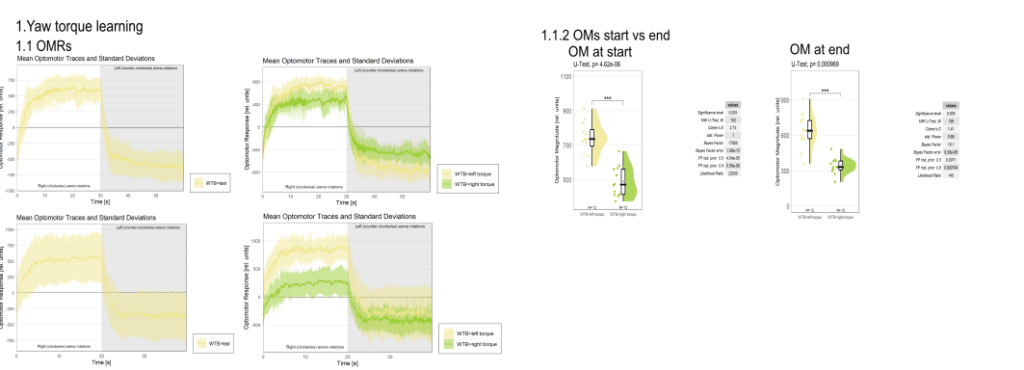
1.1 OMRs at start
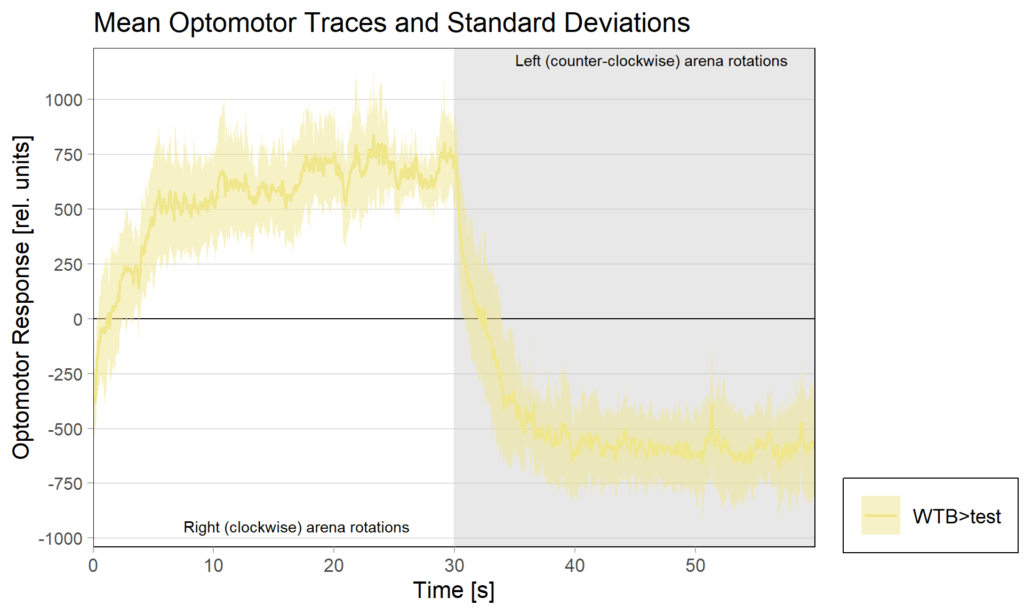
1.2 OMRs at end
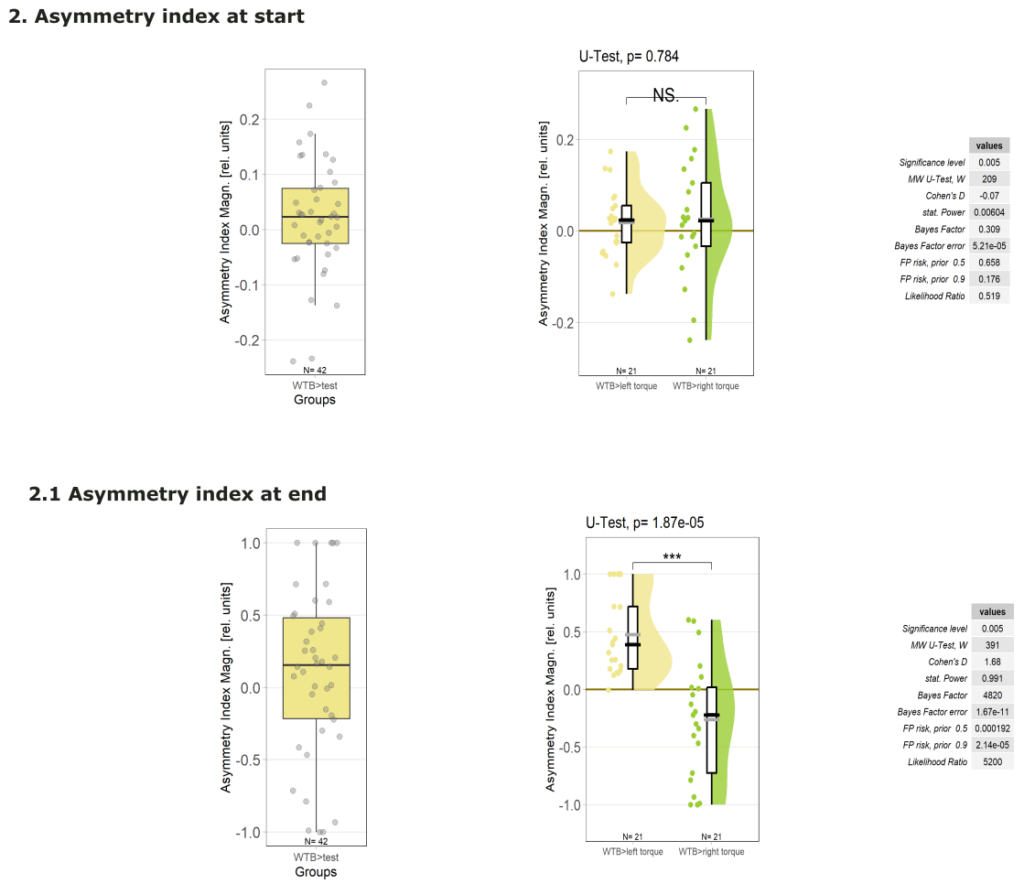
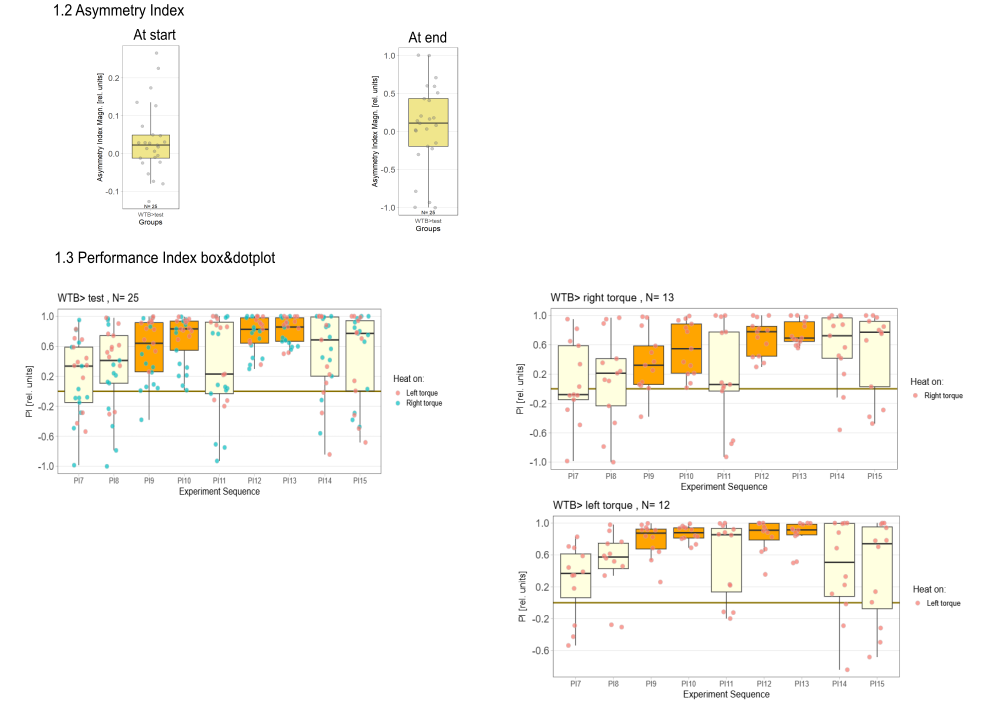
1.3 Asymmetry Index at start
1.4 Asymmetry Index at end
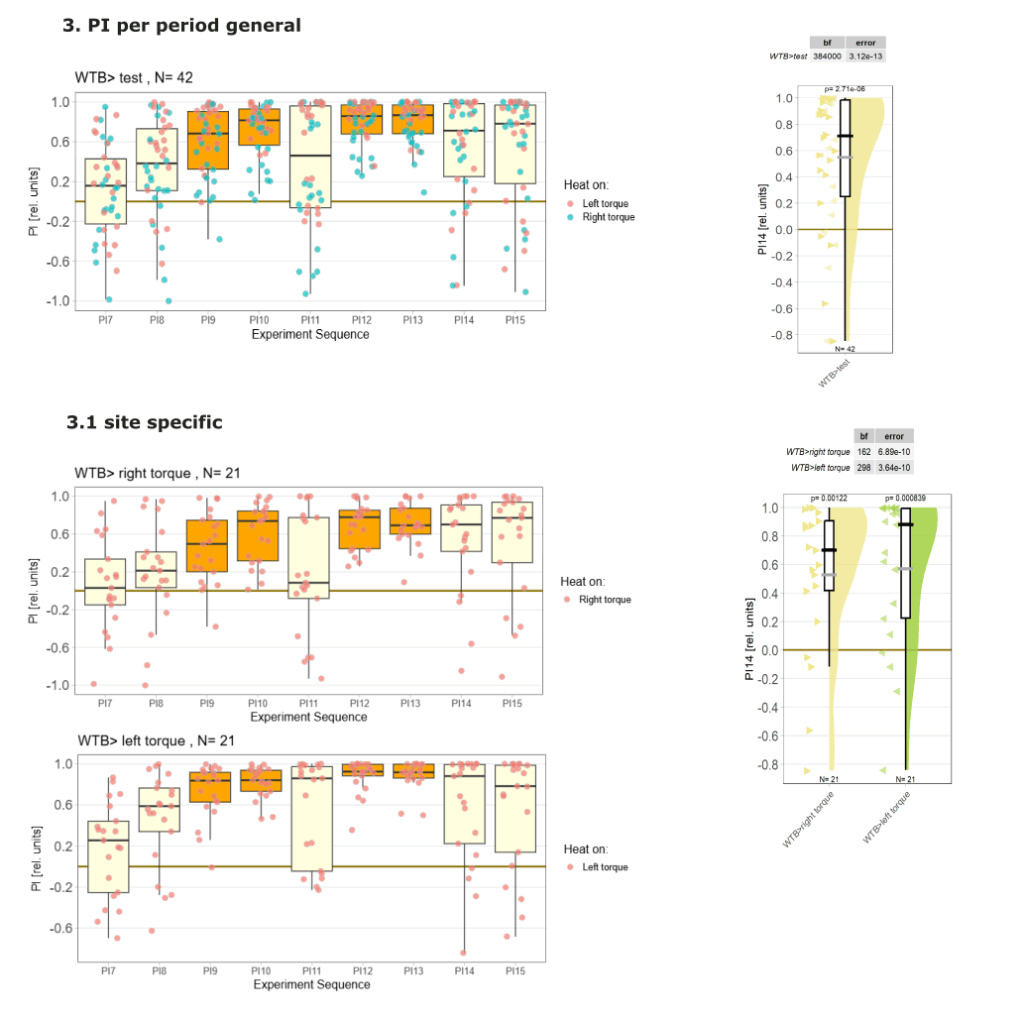
1.5 PI (preference subtracted)
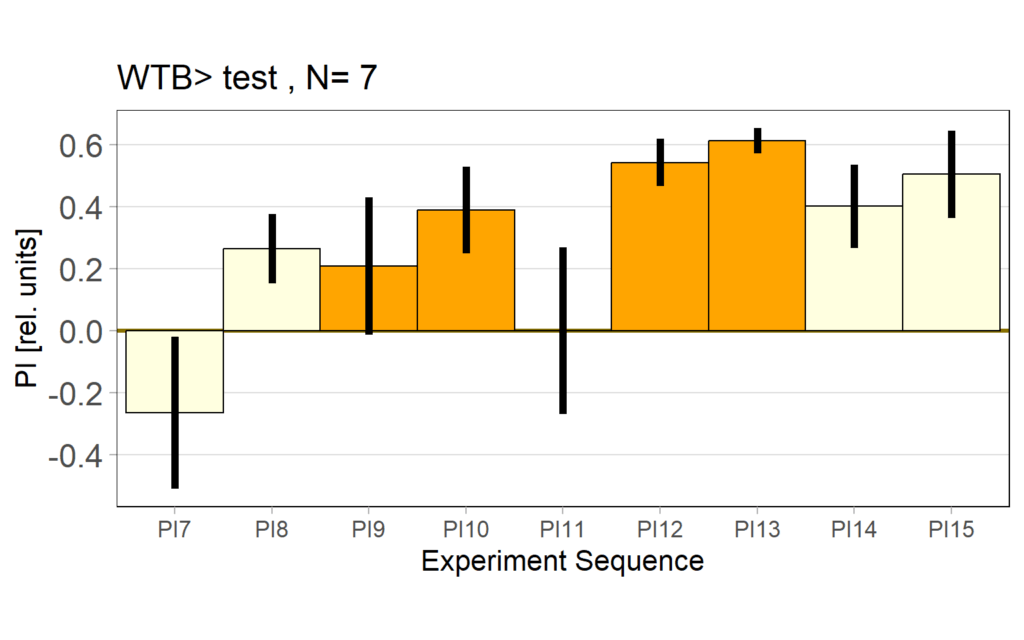
1.6 Statistics
2.
Category: Operant learning, operant self-learning | No Comments
Massacre by quality control
on Friday, September 26th, 2025 3:45 | by Björn Brembs
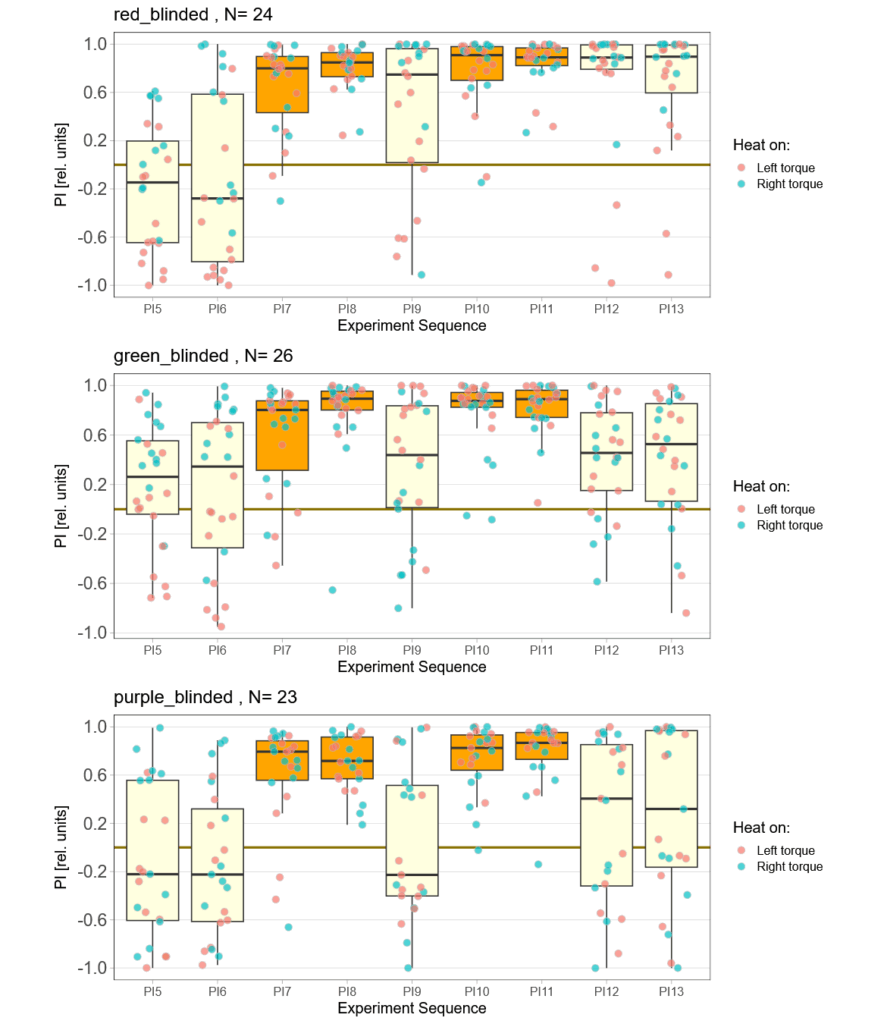
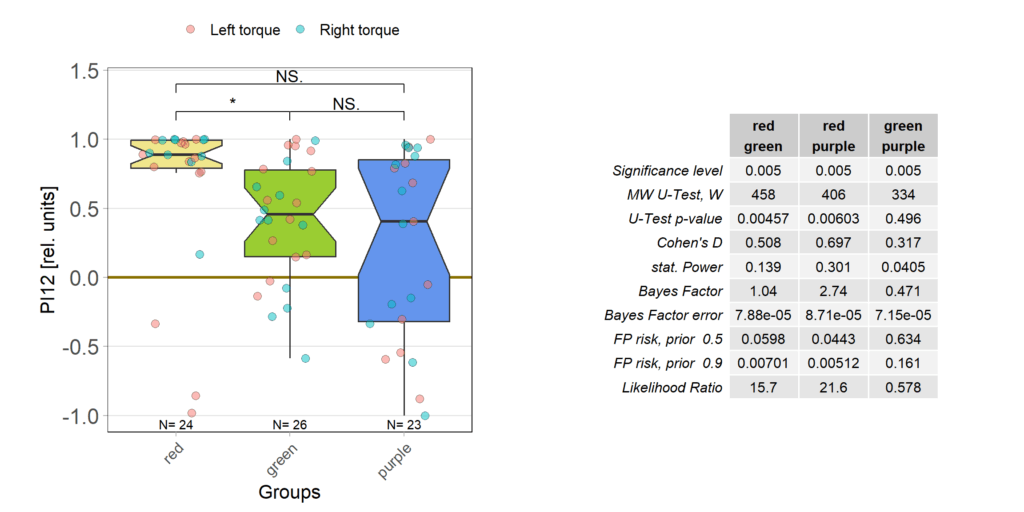
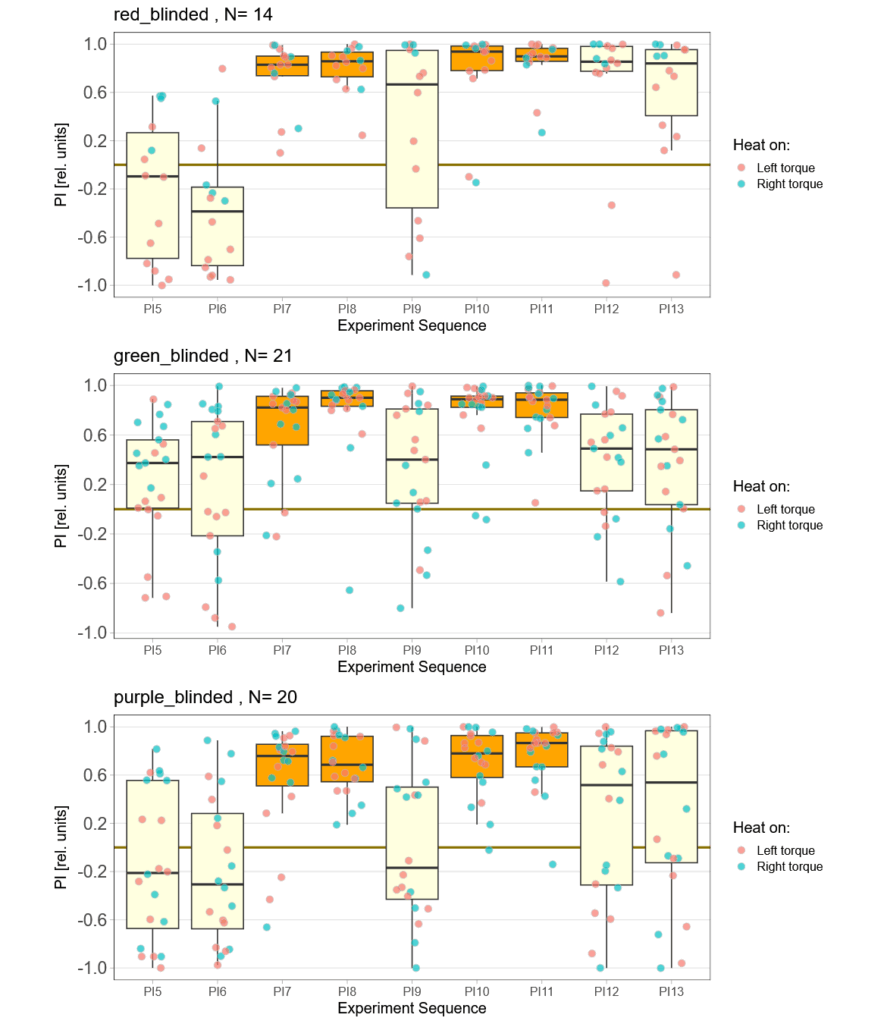
Finally found some time to go through all the individual fly data. All groups lost some flies that had to many or too long flight pauses, strong preferences already before training or some other screw-up during the experiment that I either failed to notice or only showed themselves in this quality control phase. And it was the red group, where I had thought everything just worked so fine, that lost most flies. More than a third of all the flies I had tested in this group failed the very basic quality control criteria – double the number of any other group. This is very annoying as it means there now are a lot more flies for me to test. Nothing to do about that, than to carry on. Here is the result after the massacre, where red lost 10 flies, green lost 5 and purple lost 2:
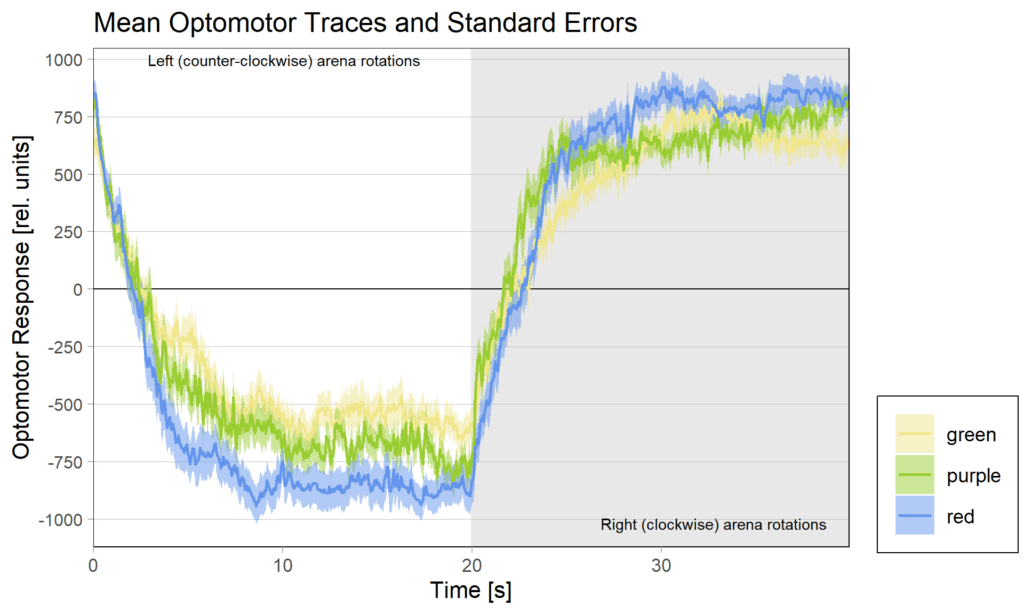
But even with so many flies removed, the red group still has the flies with the strongest optomotor response:
Category: Operant learning, operant self-learning | No Comments
Collecting ellipsoid-body self-learning data, week 2
on Wednesday, September 17th, 2025 9:23 | by Björn Brembs
Now two weeks of data collection, but so far no time for quality control. This means a bunch of flies will likely drop out after I have been able to check for the quality of the reocrdings. So far, one group (red) seems to learn really well, while the other two are too early to tell.
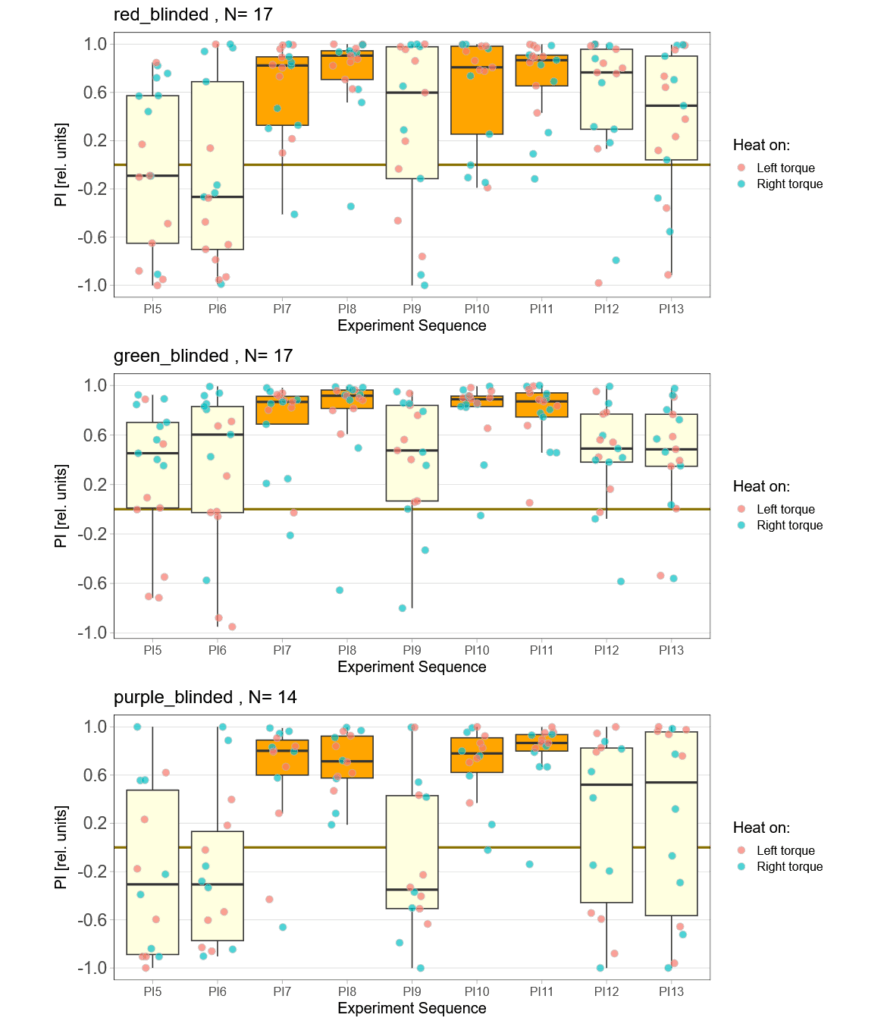
Raw data avilable on the publication server.
Category: Operant learning, operant self-learning | No Comments
Knock out of aPKC/FoxP intermediates using CRISPR/cas
on Monday, September 8th, 2025 11:03 | by Julia Schulz
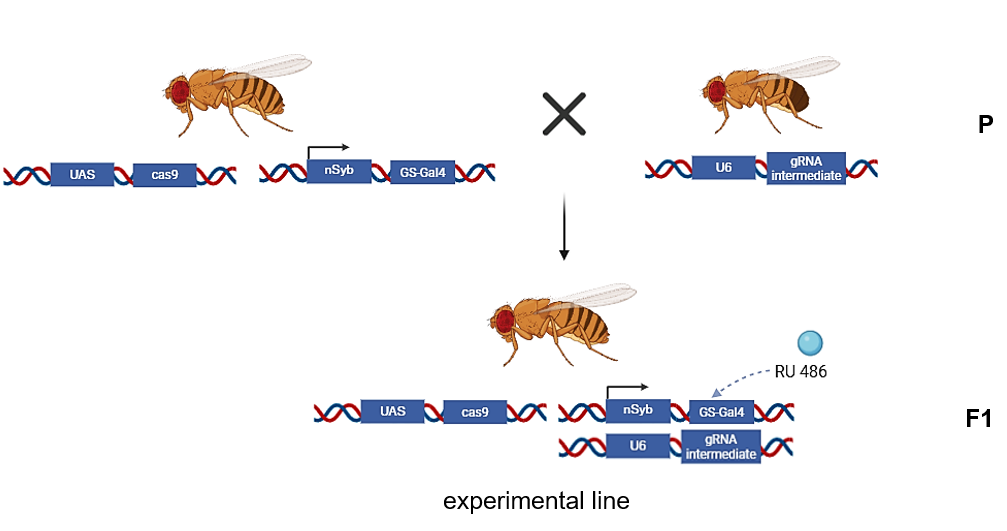
Category: Operant learning, operant self-learning | No Comments
Starting to probe the ellispoid body in torque learning
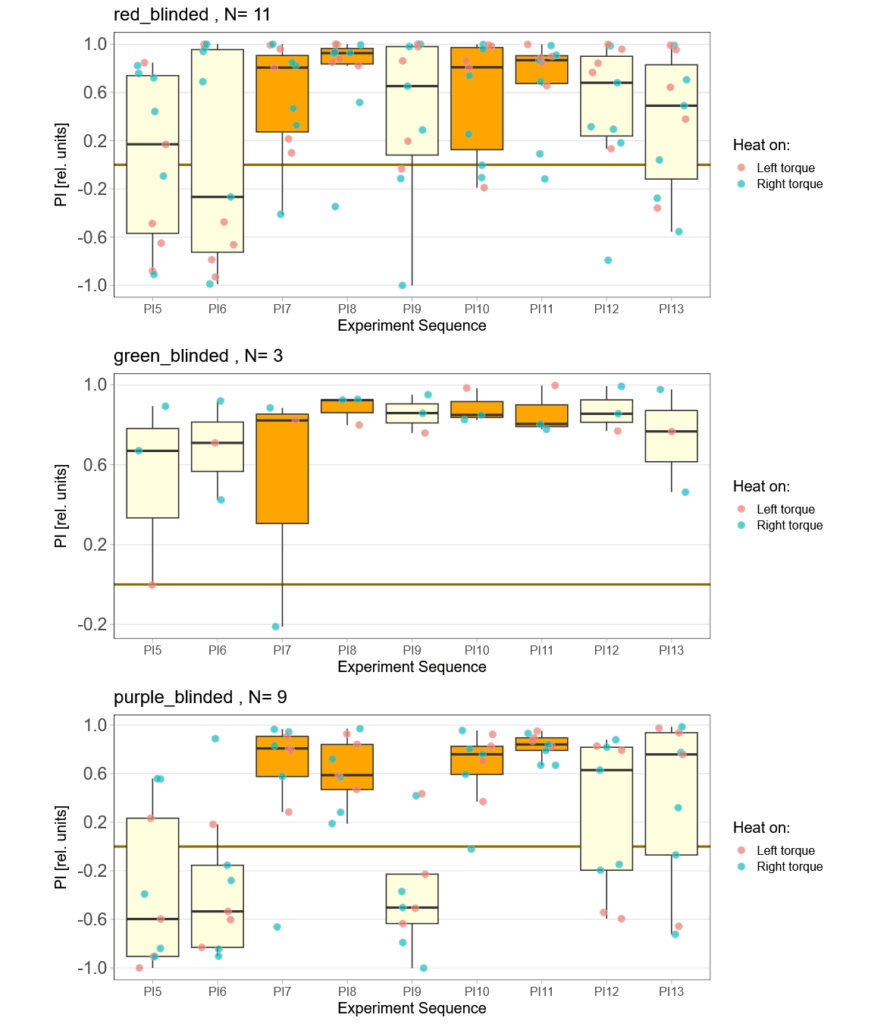
on Monday, September 8th, 2025 9:46 | by Björn Brembs
In this project, one of the two experimental groups inhibits the ellipsoid body ring neurons using TNT-E. The second uses RNAi to knock down the DopR2 receptor in these neurons and the third is the driver control. As I am blind to the groups and it’s only been one week, the data are not terribly revealing, yet.
Category: operant self-learning | No Comments
Yaw torque learning
on Monday, September 1st, 2025 12:40 | by Julia Schulz
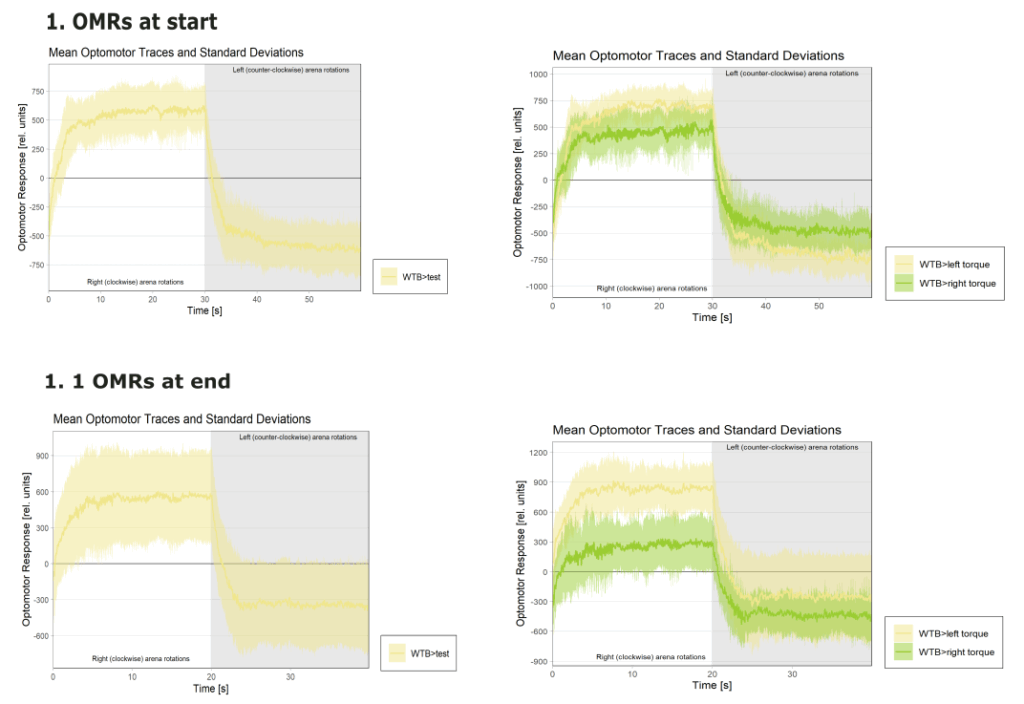
Category: Operant learning, operant self-learning | No Comments
Yaw torque learning
on Monday, August 4th, 2025 1:57 | by Julia Schulz
Category: Operant learning, operant self-learning, Optomotor response | No Comments
