Overview PubMed literature analysis for Query “Drosophila optogenetic dopamine neurons behavior”
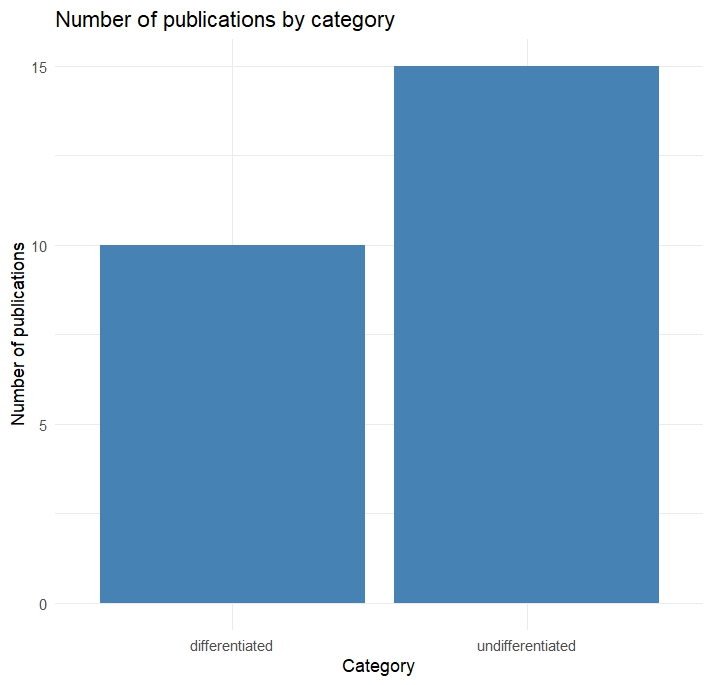
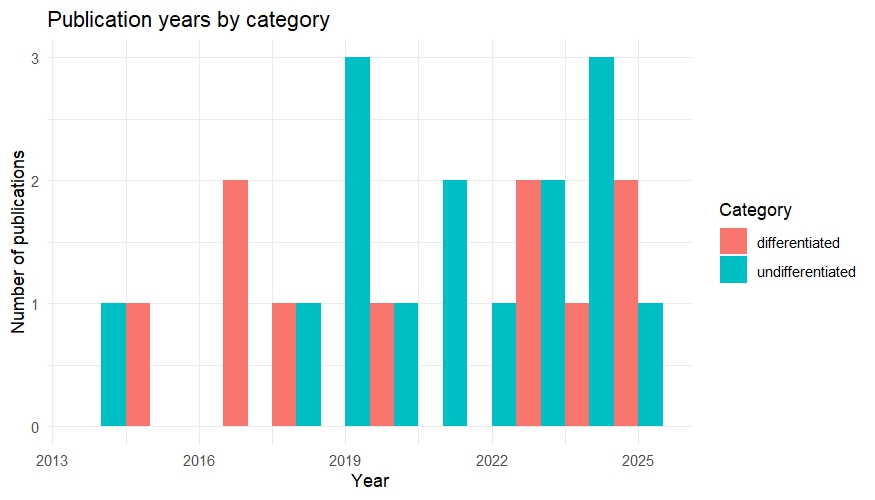
Since this will be a main focus of my master thesis I started a literature analysis to check how the idea of a possible “reward system” in Drosophila is addressed. Based on how authors address this topic, I categorized the results by either “differentiated” or “undifferentiated”. In this case undifferentiated means the authors were addressed certain sets of neurons as “reward neurons” or “punishment neurons”, although they could never be classified in this broad sense. For example a lot of papers of the “undifferentiated” category address dopaminergic neurons from the PAM cluster as “reward” neurons, since flies formed appetitive olfactory associative memories, when the CS (odors) were paired with optogenetic activation these neurons. Since PAM neurons are activated during sugar detection it makes sense that they can substitute for reward, but that doesn’t make them “reward neurons”. Real “reward neurons” would activated in any instance of reward, independent of whether it is sugar, mating or water. Many authors do not address this issue properly. Although this analysis is not finished yet I created two plots to visualize the general trends:
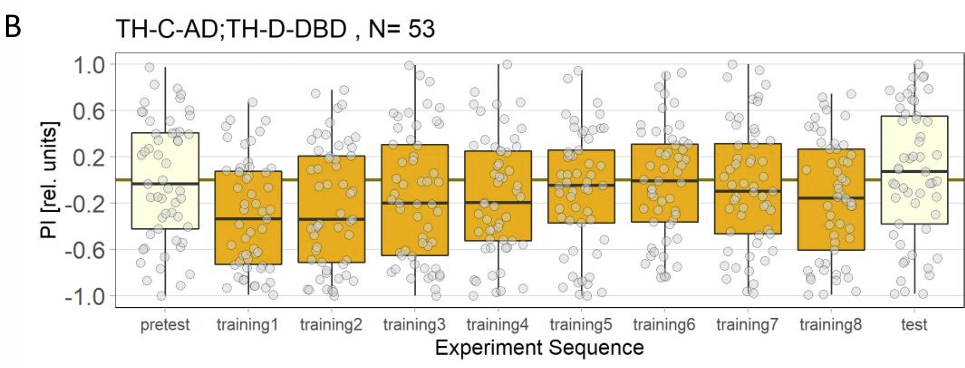
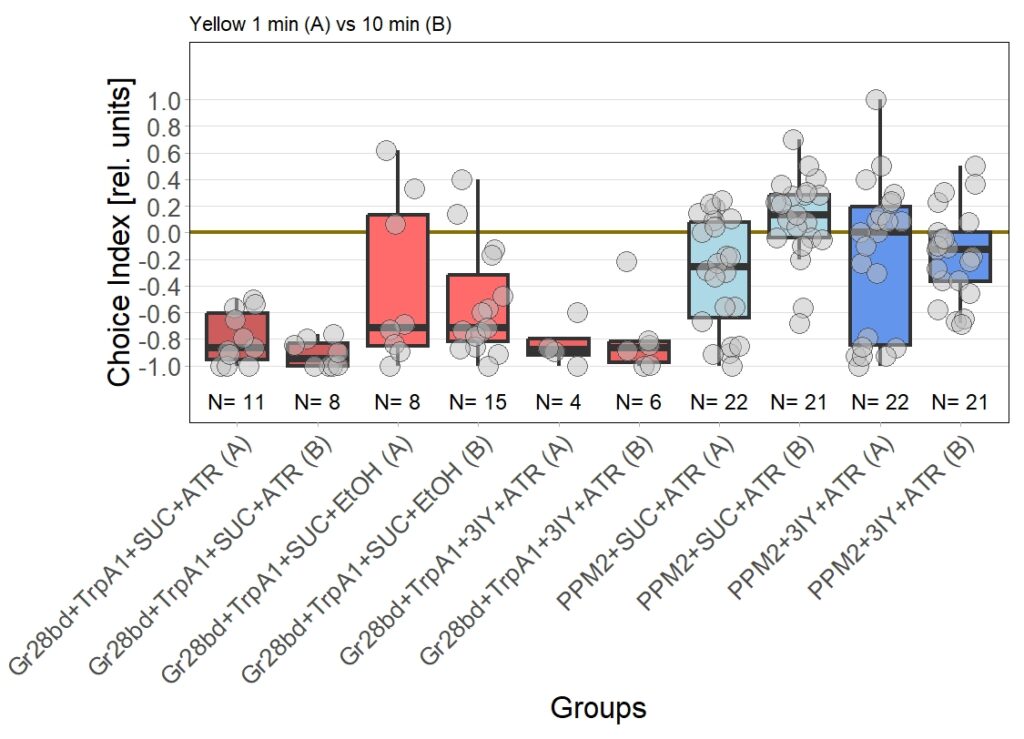
Final results of yellow light T-Maze experiments with dopamine depletion using 3IY
In previous experiments we tested flies expressing the optogenetic CsChrimson channel in PPM2 neurons. We observed mild avoidance at 1 minute, which decreased over the time course of 10 minutes, even leading to positive choice indices. To verify whether the observed effect was instructed by dopaminergic signaling, I depleted flies of dopamine using the competitive tyrosine-hydroxylase inhibtor 3-Iodo-L-Tyrosine, and tested flies again in red and yellow light T-Maze, for 1 and 10 minutes. The figure below shows the results for the yellow light T-Maze. Although we can see the same trend from avoidance in the beginning to mild approach after 10 minutes, this effect was not significant. For the 3IY treated experimental groups, we can assume that the general effect mediated by these neurons seems to be absent, when animals are depleted of dopamine.
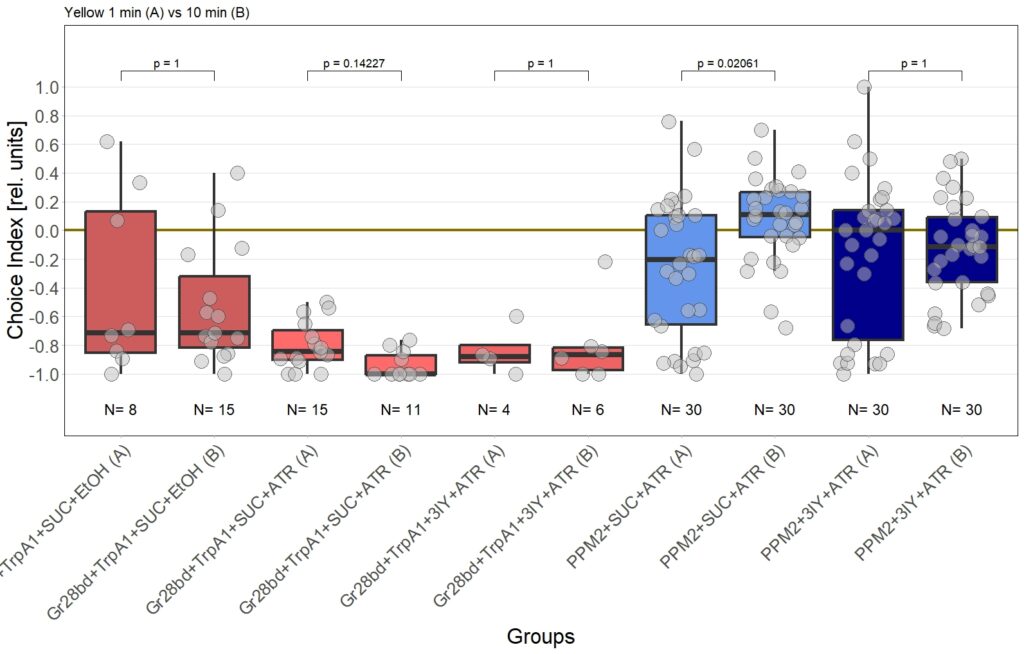
Although I could not observe a significant effect here, I plan to follow up these experiments with yet another set of T-Maze experiments. This time I plan to actively activate the neurons prior to testing. I hypothesize, that prolonged activation of the neurons might affect their valence for the animal and therefore expect animals which experienced this activation to show differences in 1 minute testing.
Current T-Maze results and power analysis
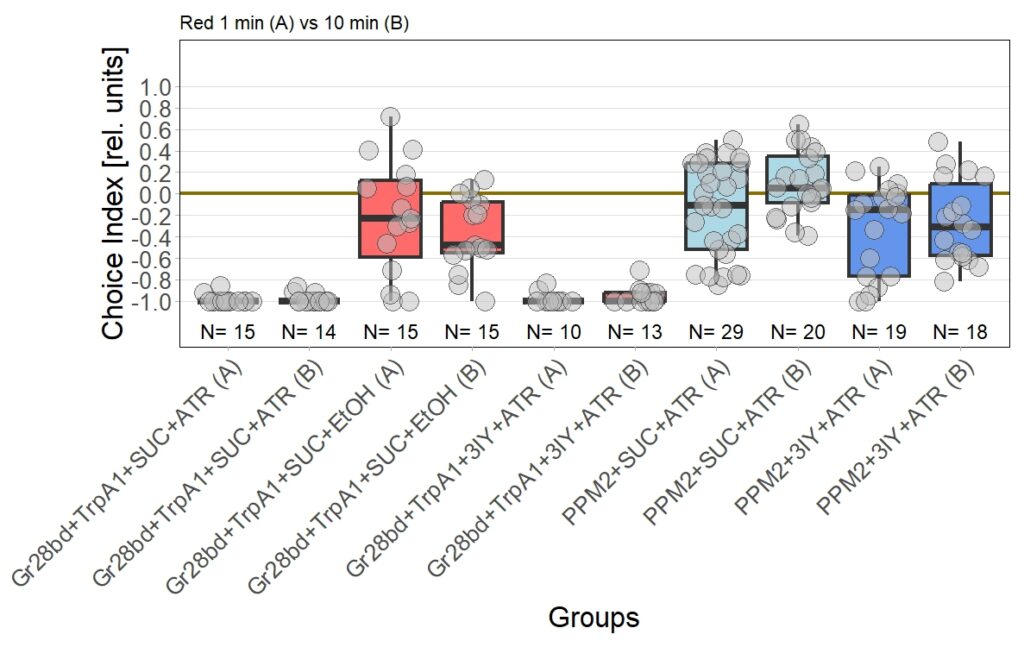
I am currently still performing T-Maze experiment with red light, to check for dopamine dependency of the effect I observed in the original 1 vs. 10 minute T-Maze experiments using yellow light and PPM2 flies.
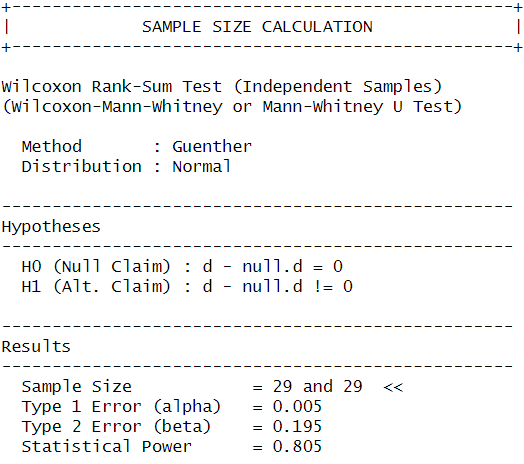
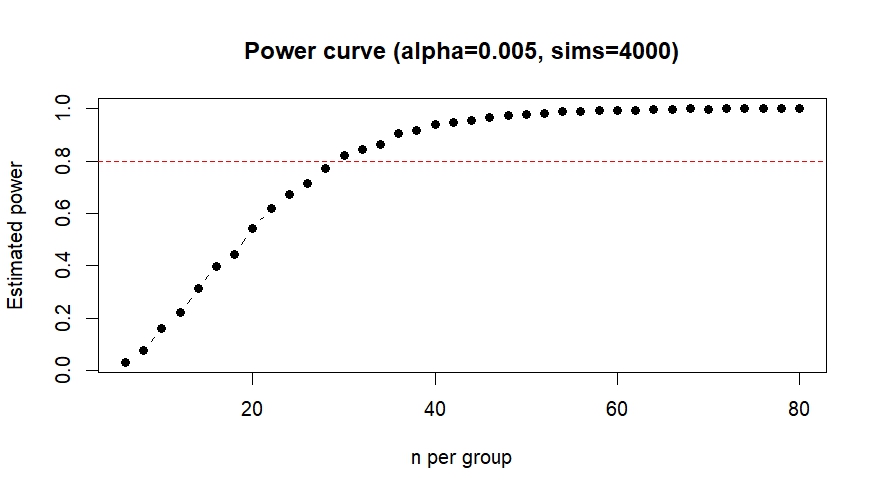
For now I planned to aim for a sample size of 30 experiments per experimental group, with the justification that this was also was I aimed for in the original screen. To see whether this sample size is sufficient to verify the effect (or it’s absence) I performed a power analysis and based it on the previously observed effect size.
Cohen’s d (from original screen) = -1.023 (large effect)
I used the power.np.wilcoxon() function from the pwrss package to calculate the sample size needed to achieve 80% statistical power with an alpha level of 0.005.
This confirmed that I would need at least 29 observations per group to detect the same effect as observed before, so I will keep aiming for a sample size of 30. Since I was unsure whether my analysis was correct I performed a second power analysis in R with an analysis script provided by ChatGPT, which calculated the sample size using simulated drawings from my original data. This analysis also resulted in a target sample size of 30 so I am fairly confident my calculations were correct.
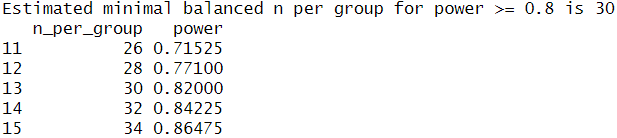
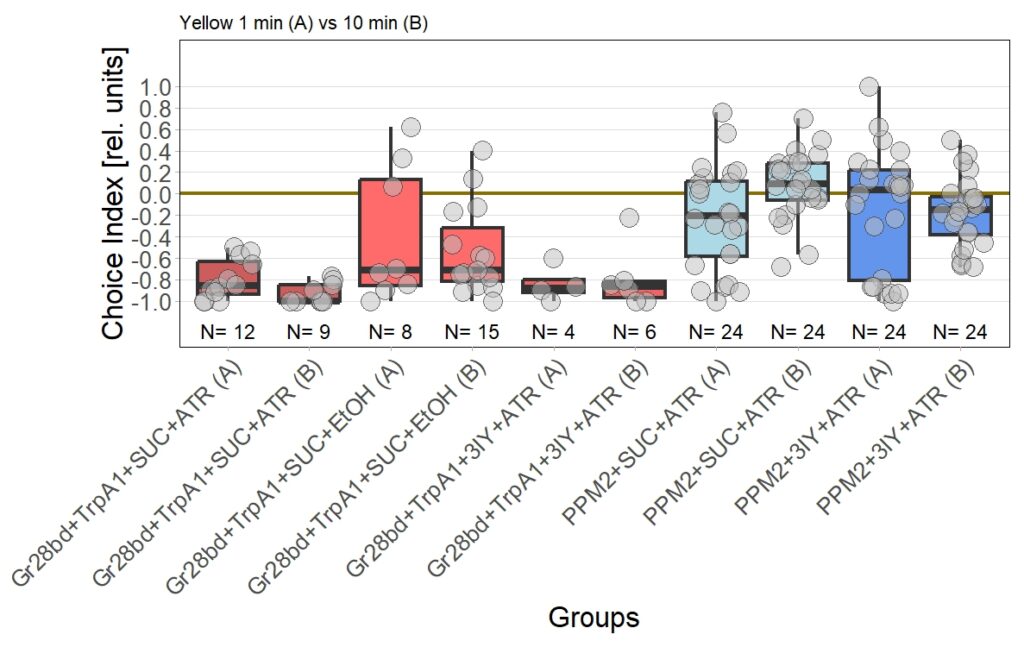
Yellow T-Maze Results
I performed the first set of T-Maze experiments, which included 3IY treated flies, with red light. In this experiment I could nicely reproduce earlier results in PPM2 flies only treated with ATR. Flies initially showed weak avoidance of optogenetic stimulation, and developed a weak approach-behavior over the time course of ten minutes. In the 3IY treated flies I found similar avoidance after 1 minute of testing but, interestingly, flies kept the same level of avoidance also for a choice time of 10 minutes. This indicates that initial avoidance might be independent of dopamine, but prolonged or repeated release of the neurotransmitter from PPM2 neurons might lead to circuit changes, weakening avoidance behavior, potentially even changing it to approach.
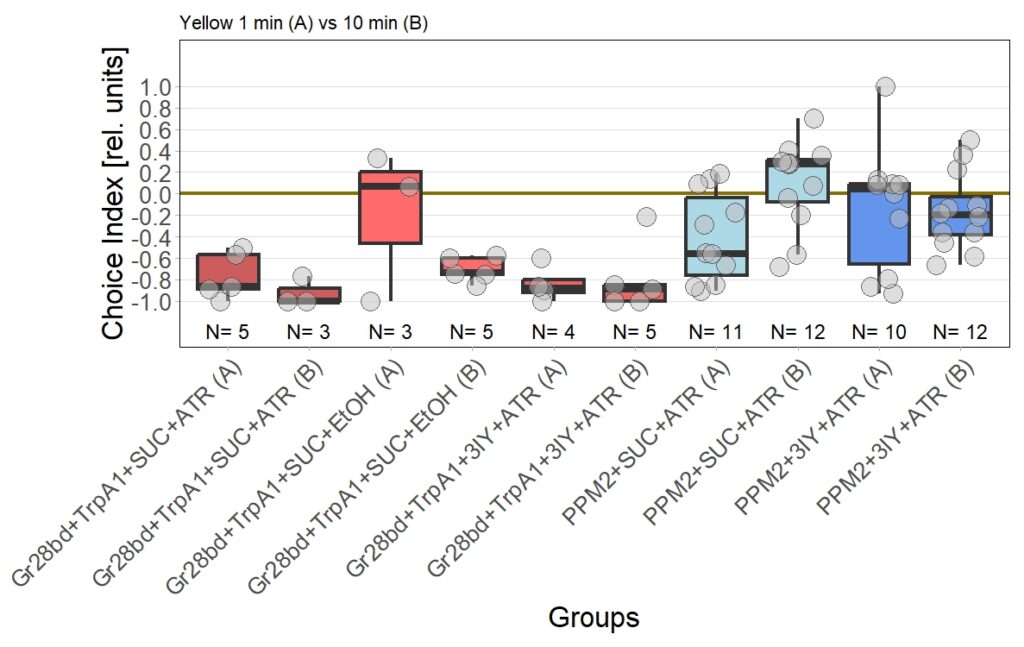
The new set of experiments uses yellow light instead of red light. This experiments are especially interesting, as I observed stronger effects for the experimental group for yellow light.
There are two main points to discuss about the data.
First, the negative control (Gr28bd+SUC+EtOH) … I was hoping that I solved the problem with the avoidance in flies that were not treated with ATR. These flies should not avoid optogenetic stimulation since without the chromophore, there should be no, or at least very weak, activation of the CsChrimson channel. Even if the sample size of 5 is rather low, it is a bit worrying that when these flies were tested for 10 minutes ((Gr28bd+SUC+EtOH (B)), they show avoidance comparable to control flies that were treated with ATR and tested for 1 minute ((Gr28bd+SUC/3IY+ATR (A)).
On the other hand, the experimental groups look pretty good. For now, I was not only able to reproduce results from the first 1 vs. 10 minute T-Maze testing with yellow light, it also seems that 3IY-treated PPM2 flies show the same phenotype as when tested in red light.
For the next few weeks I will have to increase sample sizes, aiming for 30 for each of the experimental groups. I will only include a few control groups treated with ATR, as the effect size here seems to allow for a lower N. Presumably, I will include more untreated control flies, to see whether the avoidance will persist or if the current results simply arise from the low sample size.
Update 28/11/25
Proceeding with T-Maze experiments
After pausing T-Maze experiments because of the issues with my negative control, I should now be abled to proceed.
Since it’s been a bit more than 2 months since the last results, this was the state of the experiments back then:
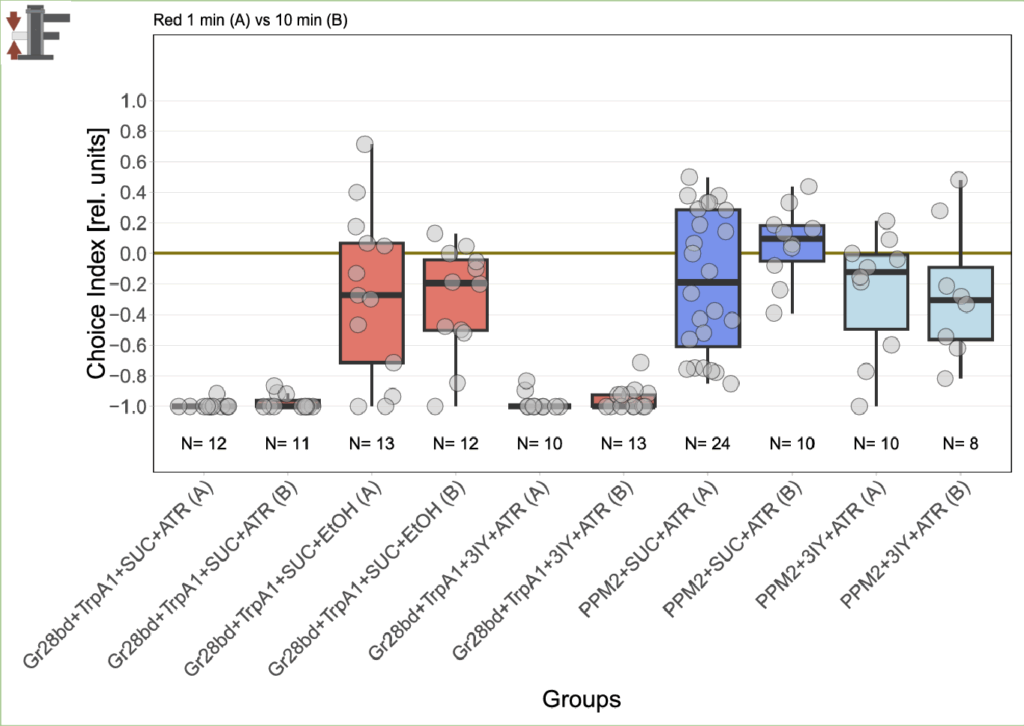
Now I added the following results:
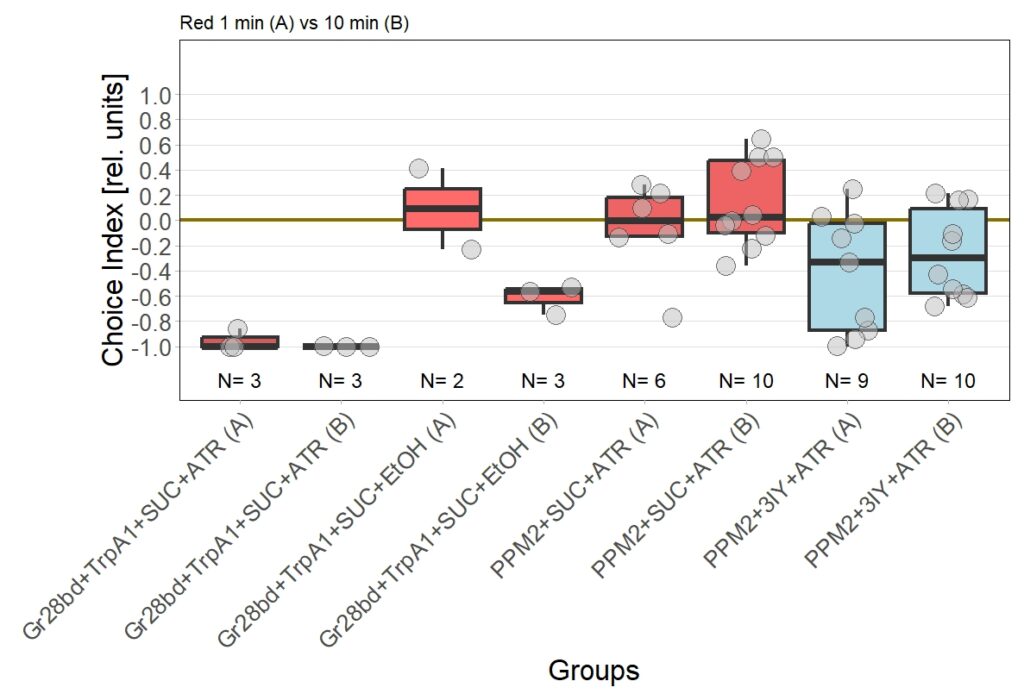
Leading to an updated version of all results:
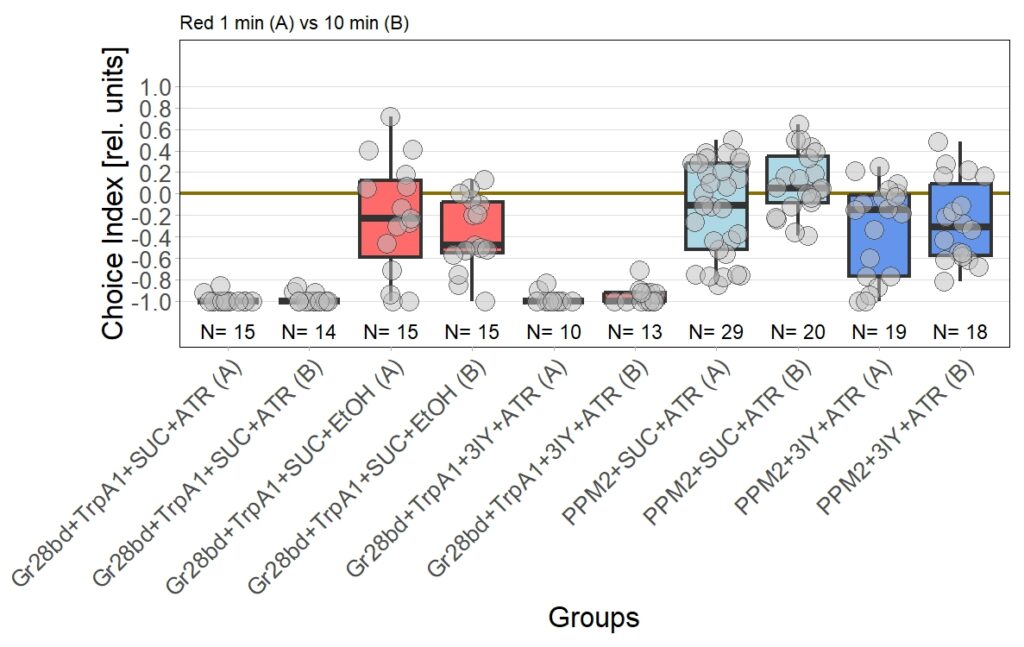
Almost there
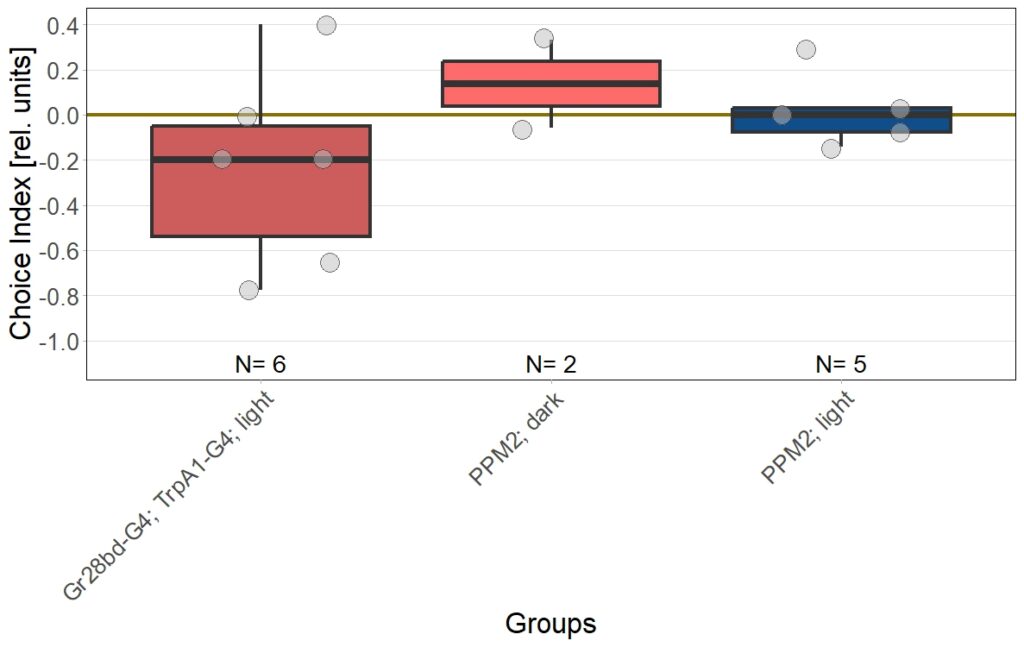
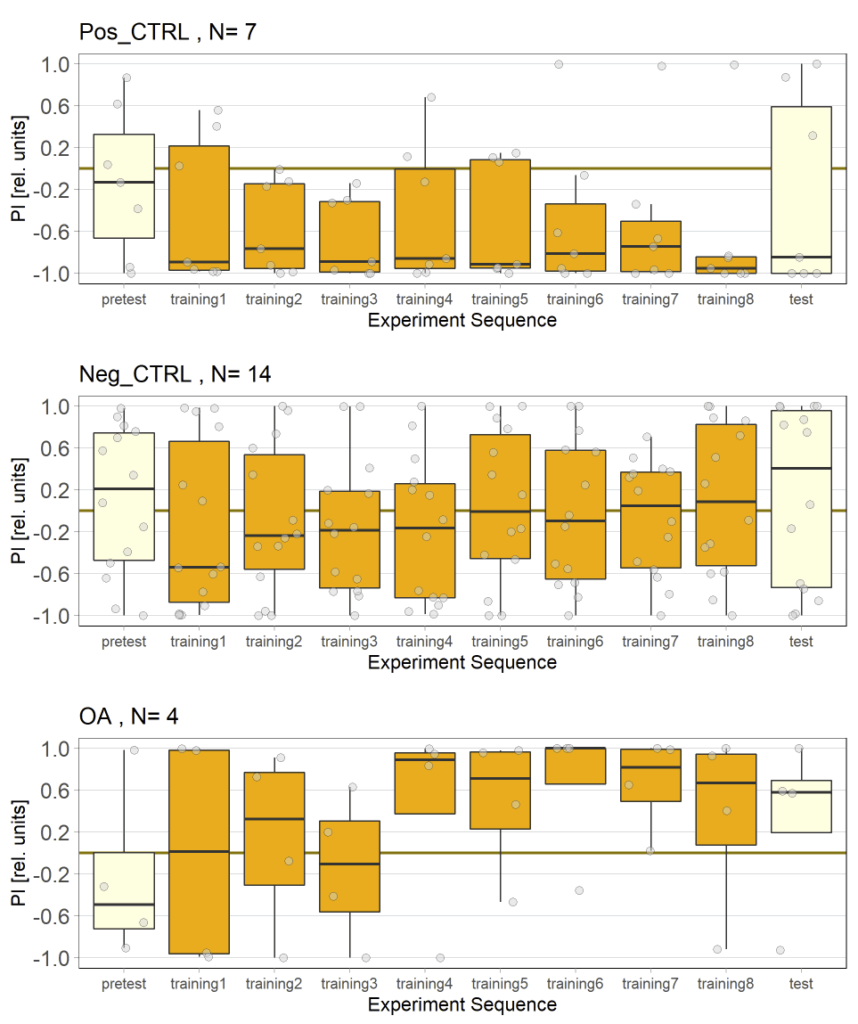
In this set of JoyStick experiments, I tested flies expressing the CsChrimson channel in OA-VuMa octopaminergic neurons, which are the Drosophila counterparts to the honeybee OA-VUMmx1 neurons. There are still a few flies left, but for now activation of these neurons does not seem to mediate any strong innate valence. There may be a slight tendency for approach in the alter periods but it might also be by chance, since we can already observe positive values in the pretest.
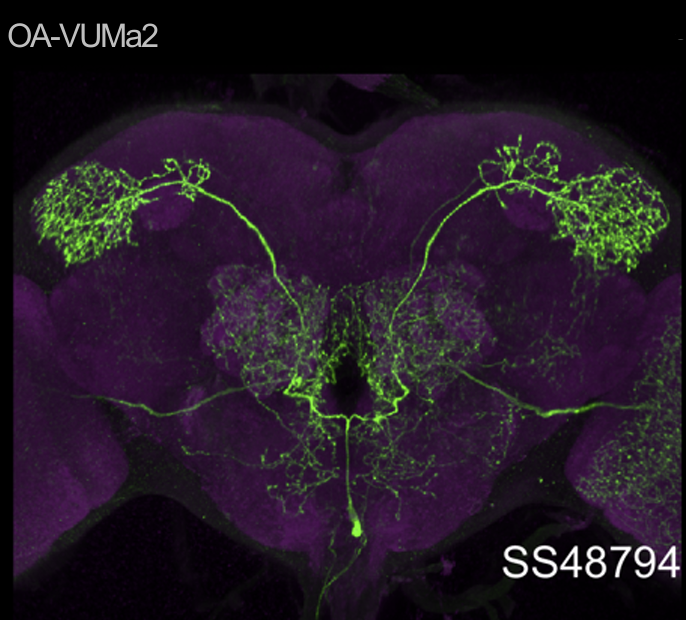
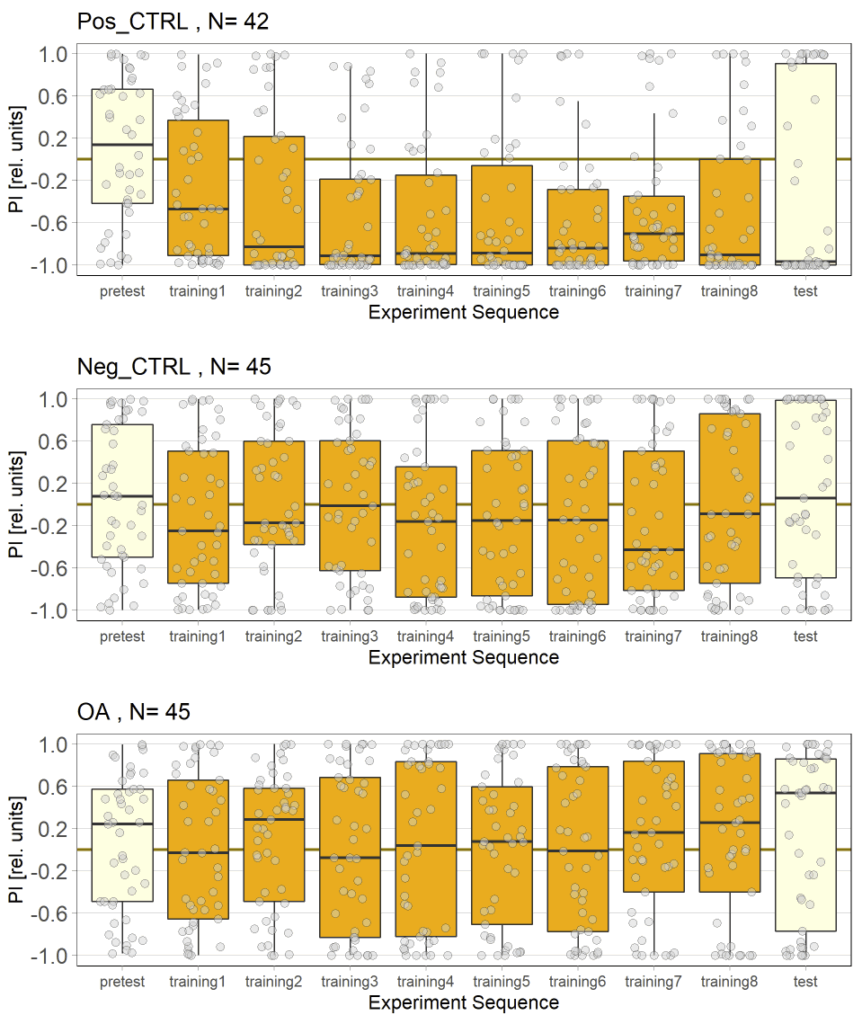
Updated results from JoyStick experiments with the “new” control driver line (and some OA flies)
Unfortunately I am still dealing with the problem that flies from my negative control group avoided optogenetic activation, even when their optogenetic channel should not work without ATR supplementation. To tackle this problem I used a “new” effector line (I prepared a new stock from our stock collection) for crossings and tested the offspring, without any improvement concerning the avoidance. Unfortunately the “new” effector line I tested turned out to have lost its “NorpA” mutation which would ensure that the male offspring is blind, thus should rule out any phototaxis-bias. Since flies still avoided the light, a) the effector line does not seem to be the problem and b) the ability to see does not seem to affect the flies behavior in the JoyStick setting.
As a next step I targeted the driver line, as maybe a mutation might have lead to an increase in Gal4. Since we always observed a slightly negative values for our negative control group, hinting that at least some residual activation of the CsChrimson channel is possible even without ATR, an increase in Gal4 might lead to a higher expression of the optogenetic channel.
Residual activation + More channels = More residual activation = Our observed avoidance???
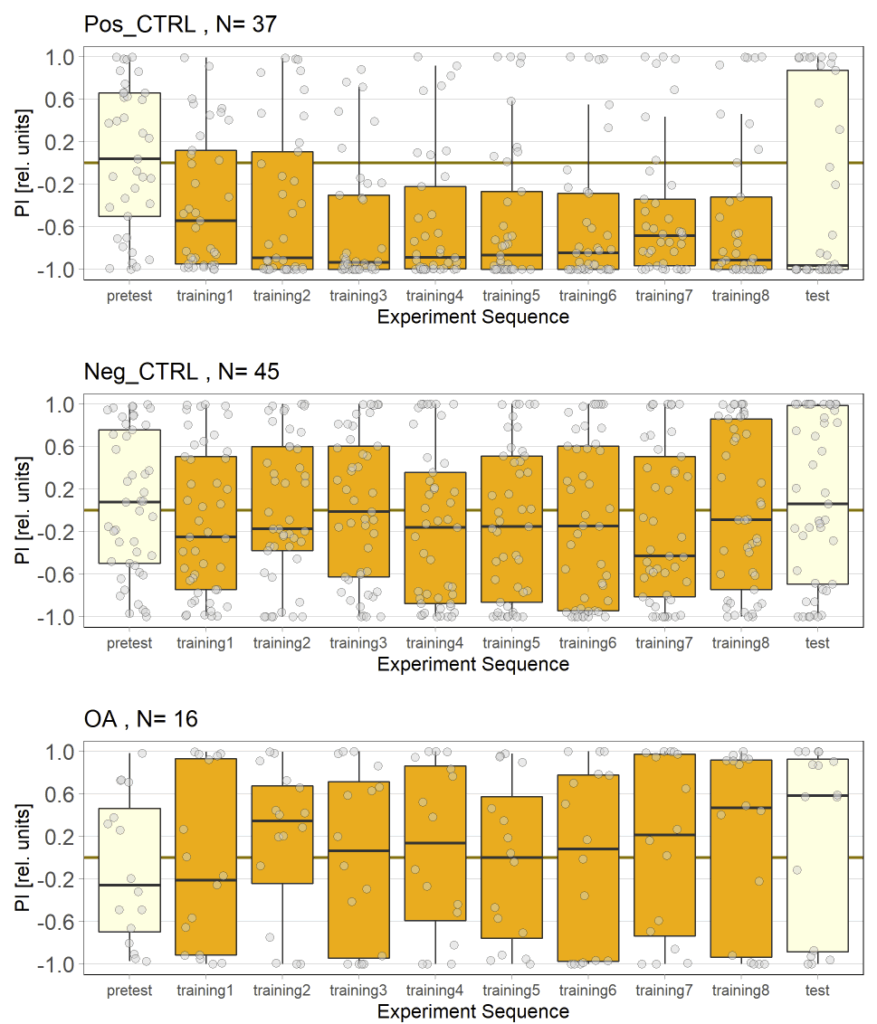
So positive control looks good, negative control is still slightly negative but to an extend that I would consider neglectable.
The additional group here called “OA” are OA;VuMA2 x NorpA, 20xUAS-Chrimson flies. It’s way too early to make any assumptions from the current data but I am looking forward to the results for this group.
Update 27/10/25: Added more flies
Trying to fix the negative control
My previous JoyStick and T-Maze experiments revealed problems concerning our control groups. While control flies that were fed with ATR supplemented food avoided the light nicely, we also observed avoidance in our negative control who did not receive any ATR. In our experiments we use the CsChrimson channel, which should need ATR to be functional.
My hypothesis for why also our negative control now shows avoidance was, that some mutation in the gene encoding for the CsChrimson channel might have affected its sensitivity and now there was at least some residual activation, even without ATR.
As an attempt to identify the underlying problem I crossed new control flies, this time using NorpA;20xUAS-Chrimson flies from our stock. If there had been a mutation in the flies I used earlier the new flies should not show avoidance.
As of now, the results are hard to interpret. There seems to be also avoidance with the new flies, however this might also be due to low sample size. If increasing the sample size will only show more robust avoidance, we’ll have to think about other causes for the problem…
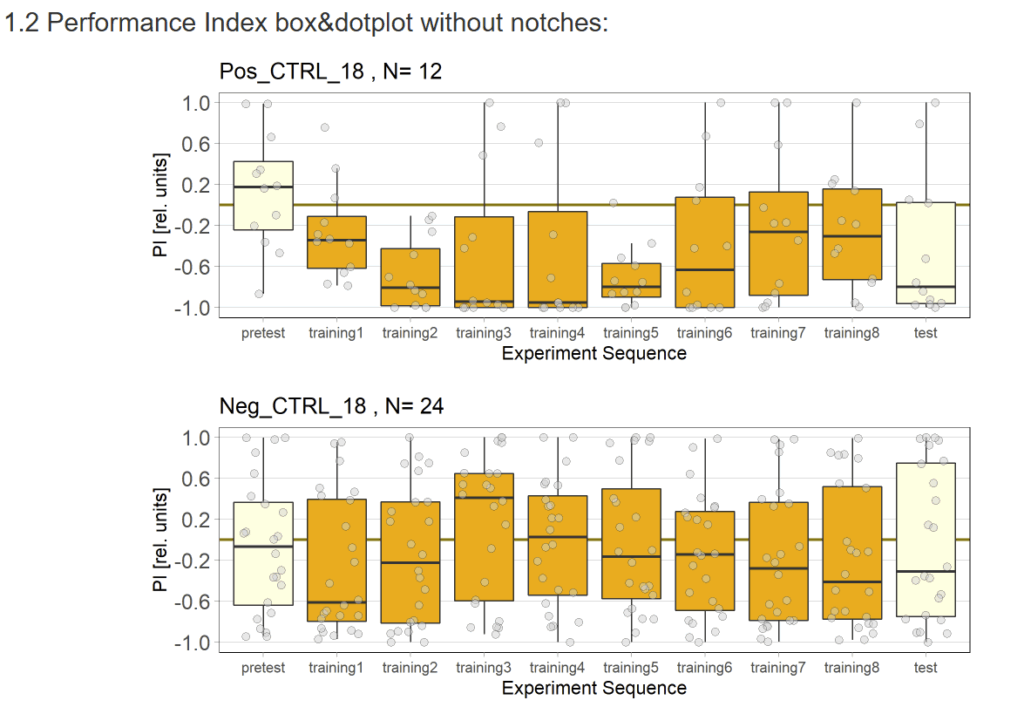
Update 17.09.25:
The flies used in the previous experiments unfortunately turned out to be not blind. I conducted a new set of experiments, this time with blind flies, and I also changed the light used to red light, at a peak intensity of 500 Lux.
With this setup the results look more like what we would expect from our negative control. Of course the sample size is too low for me to really be able to tell whether the negative control will not show a preference.
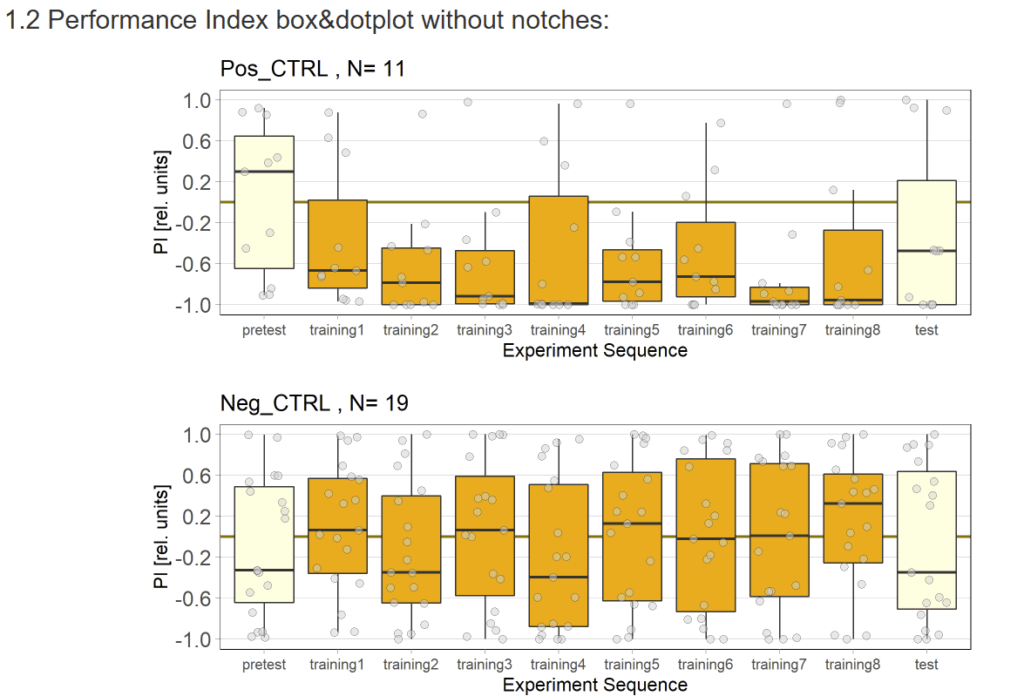
Update: 07/10/25: The results below were obtained from a new set of experiments. I reared new flies (this time the blind flies actually should be blind) and crossed them to Gr28bd-Gal4; TrpA1-Gal4 flies from our stock. If the problem was indeed the driver line, this negative control should not show avoidance. Its a bit early to say but for now it seems as I might be on the right track.
Final JoyStick results from Alisa’s trials
Control groups fed with ATR (Gr28bdTrpA1_Suc_ATR and Gr28bdTrpA1_Suc_ATR) look fine and for now the avoidance by activating heat sensing neurons does not seem to depend on dopamine. The negative control (Gr28bdTrpA1_Suc_EtOH) shows stronger avoidance than we would expect. Also PIs of PPM2 lies do not follow the same pattern as we previosly observed… My current hypothesis is that some sort of mutation led to the Chrimson channel being more sensitive to light, even without ATR. Since we rear the PPM2 flies in light until they receive the ATR this might affect their behavior.
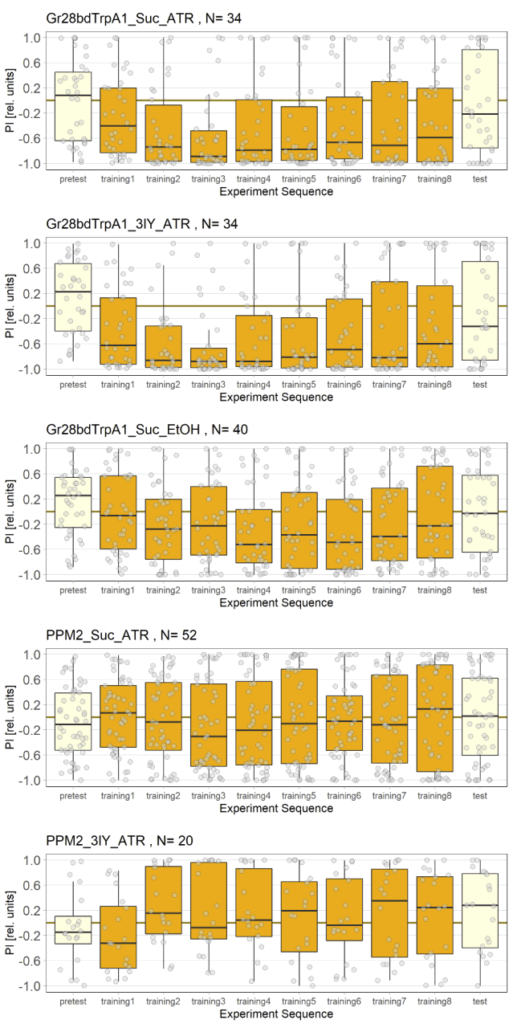
Luisa’s results for yellow light: